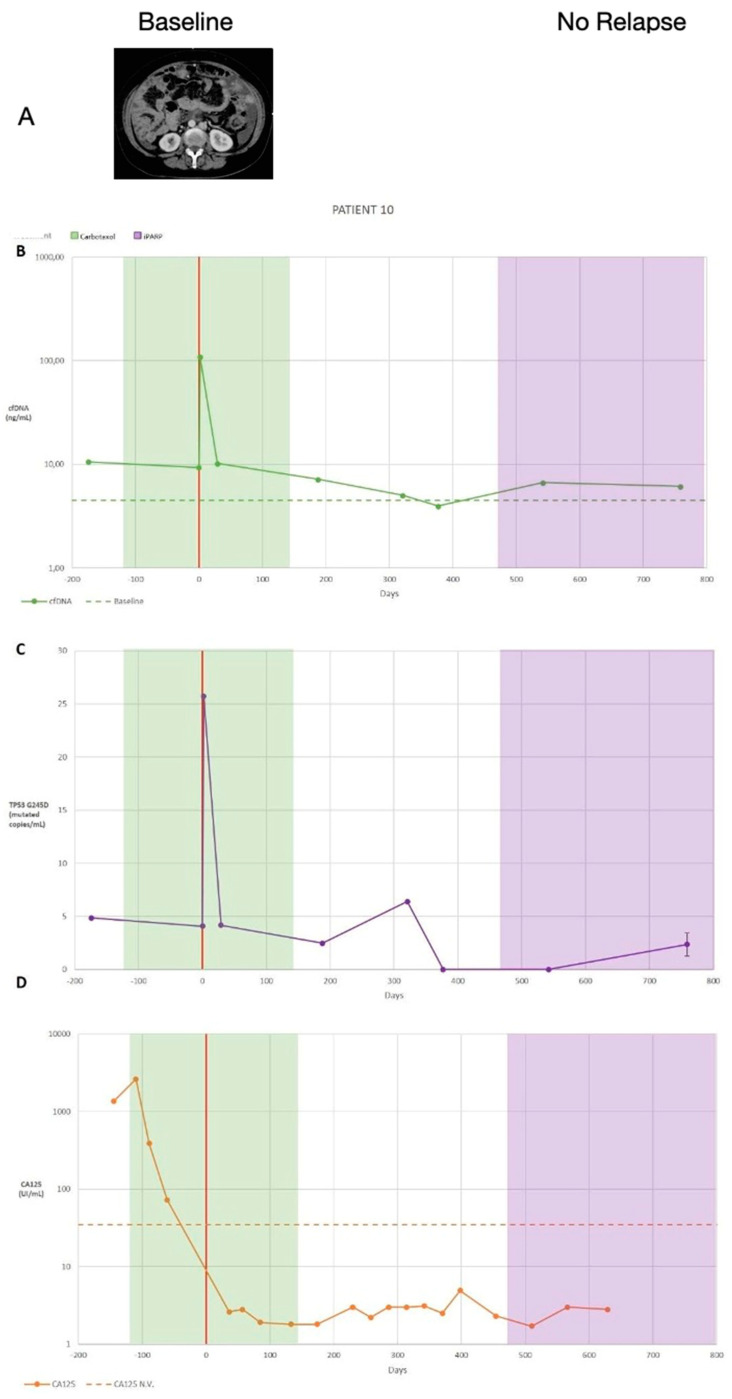
Figure 5.
(A) Summary of patient 10’s clinical information, including the treatment type and dates (treatment dates indicated by the coloured vertical stripes, and date of the cytoreduction surgery indicated by a red vertical line) as well as a computed tomography scan and summarised results. (B) Cell-free DNA (cfDNA) plasma levels are presented as ng/mL. (C) Circulating tumour DNA (ctDNA) plasma levels are expressed as the number of mutated copies/mL. Replicates were used to quantify the average TP53 G245 levels for each time point, and the error bars represent the standard deviation across replicates (n = 3); no bars indicate that the standard deviation was too low to be visualised on the scale used. The volume-adjusted limit of detection was 21.2 GE/mL for all time points. A two-tailed t-test was used to compare the ctDNA quantities between sequential time points. (D) Cancer antigen 125 (CA125) serum levels (U/mL); data from multiple replicates were not provided. * p < 0.05, ** p < 0.01, and *** p < 0.001.