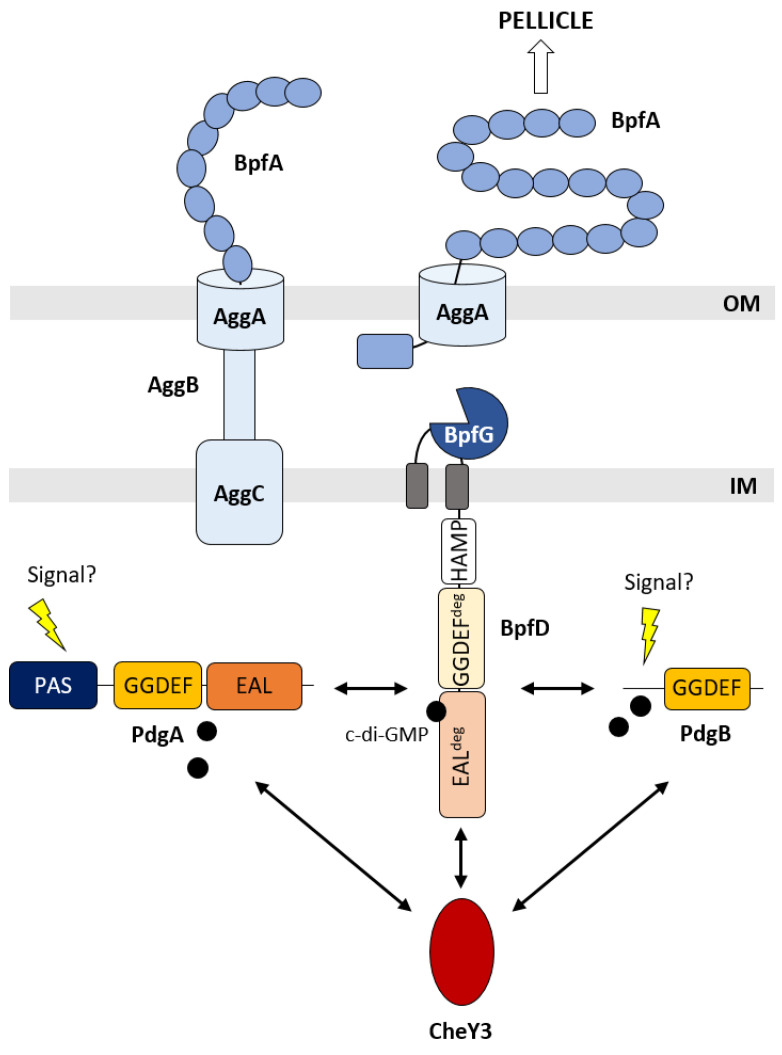
Figure 7.
The Bpf system and its partners are involved in pellicle formation in S. oneidensis. BpfA is an adhesin secreted by a type-I secretion system composed of the AggA, AggB, and AggC proteins. BpfG is a periplasmic protease. BpfD is a membrane-anchored protein with an HAMP domain as well as degenerated GGDEF and EAL domains (GGDEFdeg and EALdeg). PdgA and PdgB are diguanylate cyclases and CheY3 is a response regulator proved to be mandatory for pellicle formation. Based on our results, we propose that BpfD is a c-di-GMP effector receiving the c-di-GMP secondary messenger from PdgA and PdgB, which could be activated by yet-unknown signals. BpfD also interacts with CheY3 and therefore belongs to the complex regulatory network controlling pellicle biogenesis. Fixation of c-di-GMP on the degenerated EAL domain of BpfD probably allows BpfD to sequester BpfG, leading to the accumulation of BpfA at the cell surface and pellicle formation. The protein–protein interactions, which were experimentally proven, are indicated by double arrows. IM: inner membrane; OM: outer membrane.