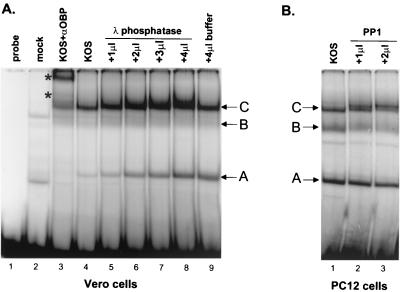
FIG. 7.
Phosphatase treatment alters the electrophoretic mobility of OBP-containing complex C bound to site I. (A) Vero cells were infected at 10 PFU/cell with KOS and harvested at 16 hpi, and nuclear extracts were prepared. Five micrograms of nuclear protein was either left untreated (lane 4) or treated with 1, 2, 3, or 4 μl of λ protein phosphatase (lanes 5 to 8) or with 4 μl of phosphatase storage buffer as described in the text (lane 9). Phosphatase-treated extracts were then incubated with the OriS site I probe as described in the text. Incubation of the OriS site I probe without extract (lane 1), with mock-infected extract (lane 2), or with KOS-infected extract in the presence of antibody to OBP (R250; lane 3) was also performed. Protein-DNA complexes were resolved in a nondenaturing 6% polyacrylamide gel and visualized using a PhosphorImager. Addition of antibody to OBP (R250) shifted the mobility of complexes A and C as indicated by the asterisks (lane 3). The intensity of complex B is weaker here than it is in Fig. 5. (B) NGF-differentiated PC12 cells were infected at 10 PFU/cell with KOS and harvested at 10 hpi, and nuclear extracts were prepared. Five micrograms of nuclear protein was untreated (lane 1) or treated with 1 (lane 2) or 2 (lane 3) μl of PP1. Binding to the OriS site I probe was evaluated by gel shift analysis as described for panel A.