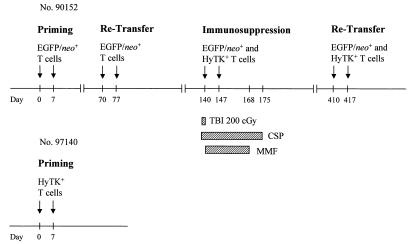
FIG. 1.
Schematic diagram of the experimental design. Each arrow indicates an infusion of 109 autologous gene-modified T cells/m2. For immunosuppression, macaque 90152 was given a nonmyeloablative dose (200 cGy) of TBI prior to the infusion of both EGFP/neo- and HyTK-modified T cells (day 140). CSP (1.5 mg/kg b.i.d. i.v.) was administered on days 137 to 175, and MMF (10 to 15 mg/kg b.i.d. i.v.) was given on days 140 to 168.