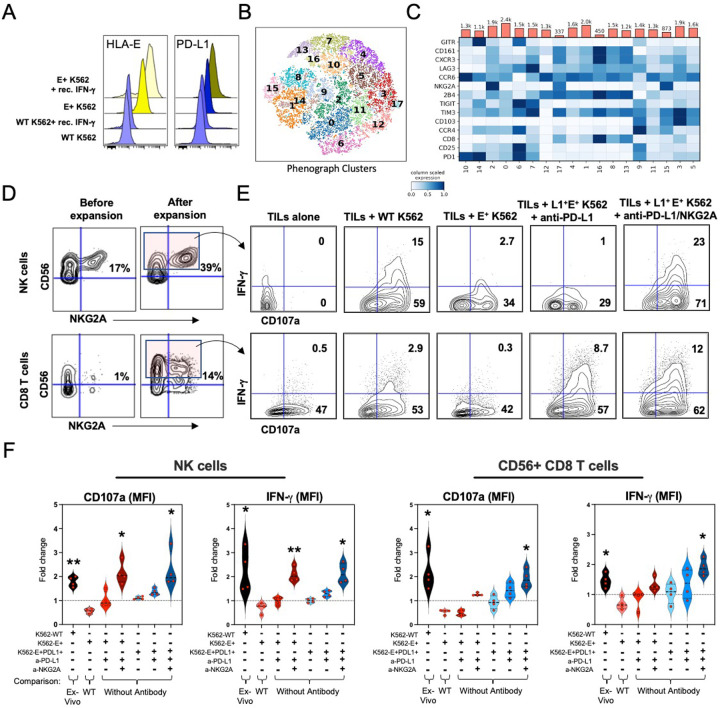
Fig. 7: In vitro combination NKG2A and PD-L1 blockade restores tumor-derived NK and CD8 T cell-mediated antitumor activity.
A, Median fluorescence intensity (MFI) of HLA-E and PD-L1 expression by wild-type (WT) or HLA-E+ K562 tumors cultured overnight in the presence or absence of recombinant human IFN-γ.
B and C, Phenograph clustering meta-analysis of tumor-derived and expanded CD56+ CD8 T cells by CyTOF before co-culture with K562 tumors showing B, tSNE analysis of identified CD8 T cell clusters, and C, expression of individual inhibitory and activating receptors by CD8 T cells and their distribution across clusters.
D, Representative CyTOF analysis of CD56 and NKG2A expression on tumor-derived NK (top row) and CD8 T cells (bottom row) profiled before (left column) and after (right column) expansion with low dose recombinant human IL-2, IL-7, IL-15 and CD3/CD28 tetramers.
E, Representative fluorescence flow cytometric analysis of IFN-γ and CD107a expression by CD56+ NK (top row) and CD8 T cells (bottom row) after 6-hour culture alone or in presence of K562 tumor lines with or without pre-treatment with anti-PD-L1 antibodies or with anti-PD-L1 + anti-NKG2A antibodies.
F, Fold-change differences between the frequencies of NK and CD56+ CD8 T cells when comparing 1271 between experimental conditions (defined by “+”). Individual comparisons are indicated at the bottom. *, p< 0.05; **, p< 0.001. P values determined by paired Wilcoxon matched rank test.