Abstract
Detarium microcarpum (Fabaceae) is a medicinal plant from the traditional pharmacopeia of Niger used against gastrointestinal disorders and dysentery. This study was designed to assess the in vitro anti-shigella, antioxidant activities, and oral acute toxicity of extract root barks of Detarium microcarpum. The crude extracts were prepared by maceration using methanol, ethanol, dichloromethane, water-ethanol (30/70 v/v), and methanol-dichloromethane (1/1 v/v). The anti-shigella activity was performed using the microdilution method coupled with the resazurin-based assay. The antioxidant activity was evaluated by the DPPH· (2, 2-diphényl-1-picrylhydrazyl), ABTS 2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid), and H2O2 assays. The oral acute toxicity was assessed following the Organization for Economic Cooperation and Development (OECD) guidelines. The extracts displayed activity against the Shigella boydii with Minimum Inhibitory Concentrations (MICs) from 500 to 1000 μg/mL. The methanolic crude extract of D. microcarpum shows good antioxidant activity with the radicals DPPH· and ABTS with inhibitory concentration 50 (IC50) at 228 and 191 µg/mL, respectively. The lethal dose 50 (LD50) of extract was up to 2000 mg/kg of body weight, and no signs of toxicity were observed. These findings supported the use of Detarium microcarpum in the traditional treatment of gastrointestinal disorders.
1. Introduction
Microorganisms are widespread in the environment, and the majority of them live on land or in water [1]. In addition to its importance in daily human life for food and nonfood needs (personal hygiene and various household tasks), water is also a means of disseminating pathogens that cause waterborne diseases in humans. Access to drinking water is an obstacle to improve people's health, despite the water supply and sanitation programmes that have been put in place. One in three people in the world (i.e., 2.4 billion people) still lives without adequate sanitation facilities, with sub-Saharan Africa the worst affected [2].
This lack is leading people to turn to alternative sources of water of dubious microbiological quality, such as water from lentic environments (wells, springs, and boreholes) and rivers. Microbial infections are diseases caused by the development, in humans or animals, of yeasts, parasite, virus, or bacteria some species of which are pathogenic. These microbial infections, which were once considered commonplace, are now classified as serious infections that can lead to a high rate of mortality and morbidity in immunocompromised patients and diabetics. Antimicrobial chemotherapy has proved highly effective against superficial infections. However, deep infections remain the most difficult to be treated because of the toxicity of systemic antimicrobials and the emergence of resistance to the most commonly used drugs. The microorganisms most frequently responsible of bacterial infections are E. coli, and the genera Shigella, Salmonella, Yersinia, and rarely Vibrio cholerae, which are only found in some specific areas such as in low-income countries [3].
In Niger, a study carried out on children aged 0–5 hospitalised or consulted at Niamey National Hospital revealed that the germs most frequently involved in childhood gastroenteritis are the bacteria from the genera Shigella and Salmonella [4]. In view of the problem caused by microorganism resistance to conventional antibiotics, there is a need for constant renewal of active ingredients [5]. Medicinal plants remain the most important sources of molecules used in the composition of pharmaceutical drugs. It therefore makes sense to continue or even intensify research in this field because these plants remain an almost inexhaustible source of biomolecules.
Ikhiri et al., 1984, and the ACCT (Agence de Coopération Culturelle et Technique) report presented by Adjanohoum et al., 1989 reported on the use of plants in traditional pharmacopoeia in Niger and listed many species, including D. microcarpum belonging to the Fabaceae family, which is widely used in traditional Niger medicine to treat microbial infections and dysentery syndromes [6, 7]. The aim of this study is to evaluate the anti-shigella and antioxidant activities of the extracts from the root bark of Detarium microcarpum. In order to know the safety of this plant, oral acute toxicity was evaluated.
2. Materials and Methods
2.1. Plant Materials
Detarium microcarpum root barks were freshly collected in March 2018 in Dosso (Niger). The plant collected was identified in comparison with an authentic sample of the herbarium specimens deposited under number 756 in the laboratory of botanic at the Department of Biology (Faculty of Science and Technology of Abdou Moumouni University). The plant material was pretreated and air-dried at room temperature for two weeks. The plant was then pulverized into powder.
2.2. Preparation of Extracts
Twenty-five grams (25 g) of fine powder was soaked into 250 mL of each solvent (methanol, ethanol, dichloromethane, water-ethanol (30/70 v/v), and methanol-dichloromethane (1/1 v/v)) and macerated for 48 hours. The set was shaken under magnetic stirring. After filtration, the solvent was evaporated using a rotavapor (Heidolph). The evaporation was done at 40°C under pressure of 175 mbar for the ethanolic extract and 337 for the methanolic extract, as well as 850 mbar for the dichloromethane extract.
2.3. In Vitro Anti-Shigella Activities
2.3.1. Preparation of Stock Solution
(1) Preparation of Stock Solutions of Extracts and Reference Antibacterials. Stock solutions of extracts were prepared at 10 mg/mL by dissolving 10 mg of extracts in 1 mL of 10% DMSO (dimethyl sulfoxide) (Sigma Aldrich). Ciprofloxacin (Sigma Aldrich) used as a positive control was prepared under the same conditions at 1 mg/mL by dissolving 1 mg of powder in 1 mL of acidified distilled water (the final acid concentration is of 0.04 N which is not harmful to the bacterial strains).
2.3.2. Bacteria Strains and Growth Conditions
Three strains Shigella boydii, Shigella sonnei, and Shigella flexneri, provided by Bei Resources, and one clinal isolate Shigella dysenteria provided by Centre Pasteur of Cameroon to the Antimicrobial and Biocontrol Unit, University of Yaoundé 1, Cameroon, were used for the anti-Shigella activity. These bacteria were kept at 4°C and revived 24 hrs prior to each assay on Muller Hinton agar (Sigma Aldrich) at 37°C. The different bacterial suspensions were prepared according to the 0.5 McFarland standard. For this, a stock suspension was prepared at turbidity 0.5 McFarland standard (corresponding to an approximate concentration of 1.5 × 108 colony-forming units (CFU)/mL) from 24-hour cultures on Muller Hinton agar (MHA) and then diluted to 106 CFU/mL for the tests.
2.3.3. Determination of Minimum Inhibitory Concentrations
The inhibition parameter of the extract was assessed by determining the minimum inhibitory concentrations (MICs) using the protocol M07-A9 described by the Clinical and Laboratory Standards Institute (CLSI) in 2012 and Abdoulahi et al. in 2023 [8, 9]. The tests were carried out in triplicates in sterile 96-well microplates. In fact, 160 μL of Muller Hinton broth (Sigma Aldrich) (MHB) culture medium was introduced into the first wells and 100 μL into the rest of the wells. Subsequently, 40 μL of a sterile solution of concentrated extract at 10 mg/mL was taken and introduced into the corresponding wells and followed by a series of 5 serial dilutions of geometry of order 2. Finally, 100 μL of a bacterial suspension with a load of 106 cells/mL was distributed into the test wells and those of the negative control. The concentrations of extracts and ciprofloxacin in the wells ranged from 1000 μg/mL to 31.25 μg/mL and from 1.95 μg/mL to 0.0153 μg/mL, respectively, and the final volume in each well was 200 µL, and the final concentration of DMSO was less than 1% with no effect on bacteria growth. The final inoculum load in each well was 5 × 105 cells/mL. The sterility control consisted solely of the culture medium. Ciprofloxacin is used as positive control. The microplates were incubated at 37°C for 24 hours. At the end of the incubation period, 10 μL of resazurin solution freshly prepared at 0.15 mg/mL was added in all wells and the plates were incubated again in the same conditions for 30 minutes. The lowest concentration at which there was no change of coloration from blue to pink corresponding to the absence of visible bacterial growth was considered as the MIC.
2.4. Antioxidant Activity by DPPH•
The antioxidant power of the crude extract of Detarium microcarpum (DeM) was estimated by comparison with a natural antioxidant (ascorbic acid AnalaR NORMAPHUR). All tests were carried out in three replicates for each concentration. DPPH· radical scavenging activity was measured according to the protocol of Brand-Williams et al., in 1995, where 10 μL of each of the ethanolic DeM solutions tested at different concentrations were mixed with 195 μL of an ethanolic solution of DPPH• (Sigma Aldrich) (120 µM) in a 96-well microplates [11]. After 30 minutes of incubation period in the dark at laboratory temperature, absorbance was read at 517 nm using a spectrophotometer UV-Vis. Inhibition of the DPPH· free radical by ascorbic acid was also analysed for comparison.
2.4.1. Determination of the Percentage of Inhibition
Free radical inhibition in percentage (I %) was calculated using the following formula:
| (1) |
A0 and A1 correspond to the absorbances at 540 nm of the radical (DPPH·) in the absence and presence of antioxidants, respectively.
2.4.2. Estimation of Reaction Kinetics
The reaction kinetics and the parameters for calculating the antioxidant activity for ascorbic acid and the DeM concentrations studied were calculated.
| (2) |
where A0 and At correspond to the absorbance at 540 nm of DPPH· at initial and steady states, respectively. At value was obtained at the steady state region where absorbance did not depict further observable decreases.
2.5. Antioxidant by ABTS Assay
Antioxidant activity was measured in accordance with the method of Rafael et al., in 2004. The ABTS (Sigma Aldrich) solution was prepared at 7 mM by dissolving the ABTS in water and treated with 2.45 mM of potassium persulphate, and the mixture is left to stand at room temperature for 12–16 h. The solution was then diluted with ethanol to give an absorbance of 0.7 at 734 nm. 10 µL of the extract or standard solution are introduced in each corresponding well, and 190 µL of the diluted ABTS solution were added. Ascorbic acid is used as the standard prepared in ethanol (96%) [12].
| (3) |
where A0 is the absorbance without sample and AS is the absorbance with sample.
2.6. H2O2 Scavenging Capacity Assay
Antioxidant activity was measured in accordance with the method of Wang et al., in 2000. The H2O2 solution (2 mM) is prepared in a phosphate buffer (pH 7.4) at 50 mM. In the 96 well plate, 18.2 μL of extract at different concentrations and or standard, 72.7 μL of 50 mM phosphate buffer, and then 109 μL of H2O2 (2 mM) are, respectively, introduced. The absorbance is read at 230 nm after 10 minutes of incubation. 50 mM phosphate buffer without H2O2 is used as a blank [13]. Hydrogen peroxide scavenging ability (in triplicate) is calculated by the following formula:
| (4) |
where A0 is the absorbance without sample and AS is the absorbance with sample.
2.7. Acute Oral Toxicity Evaluation
2-month-old NMRI mice weighing between 27 and 32 g were used to study the acute oral toxicity. The acute oral toxicity test was conducted using the “dose adjustment” method of OECD line 425 (2008) and involved testing D. microcarpum root bark extract at a single dose of 2000 mg/kg body weight [14]. The test was carried out on 6 NMRI mice. They were fasted for 24 hours and then divided as follows: control lot consisting of 3 NMRI mice receiving a normal saline solution (NaCl 9‰) at a dose of 5 mL/kg and experimental lot consisting of 3 mice receiving the extract, at a dose of 2000 mg/kg. Behavioural monitoring was performed for 6 h after administration of the extract. The mice were then fed and hydrated ad libitum. At D0, D1, D7, and D14, the mice were weighed and sampled for blood analysis. Liver (alanine aminotransferase (ALAT) and aspartate aminotransferase (ASAT)), kidney (creatine and creatininemia), and immune (red and white blood cell counts) organ balances were assessed.
2.8. Statistical Analysis
Data were expressed as the mean ± standard deviation (m ± S.D). One-way analysis of variance (ANOVA) followed by Dunnett's test was used to determine the degree of statistical significance of results. Difference was considered as significant at p < 0.05.
3. Results
3.1. Extraction Yield
The choice of this plant was based on the literature on ethnobotany in Niger, and the results of previous surveys carried out by several researchers were used. Five solvents were used to prepare five organic extracts, with extraction yields varying from 13.1 to 34.7 percent (Table 1). The extraction yields were dependent on the extraction solvent.
Table 1.
Extraction yield.
| Solvent | MeOH | EtOH | CH2Cl2 | Water-EtOH (30/70) | MeOH-CH2Cl2 (1/1) |
|---|---|---|---|---|---|
| Yield (%) | 27.5 | 26.8 | 10.2 | 34.7 | 13.1 |
3.2. Anti-Shigella Activity of D. microcarpum Root Bark
The minimum inhibitory concentrations of D. microcarpum root extracts vary from 500 to 1000 µg/mL on shigella subhasis (Table 2).
Table 2.
Minimum inhibitory concentrations of extracts.
| MIC (µg/mL) | ||||
|---|---|---|---|---|
| Bacterial strains | SB NR 521 | SO NR 519 | SF NR 518 | SD CPC |
| EDM | 500 | >1000 | >1000 | >1000 |
| DMDM | 1000 | >1000 | >1000 | >1000 |
| DDM | >1000 | >1000 | >1000 | >1000 |
| EWDM | 500 | >1000 | >1000 | >1000 |
| MDM | 500 | >1000 | 1000 | >1000 |
| CP | 0.015 | 0.030 | 0.030 | 0.060 |
SB NR 521: Shigella boydii, SO NR 519: Shigella sonnei, SF NR 518: Shigella flexneri, SD CPC: Shigella dysenteria, EDM: ethanol crude extract, DMDM: dichloromethane-methanol crude extract (1 : 1), DDM: dichloromethane crude extract, EWDM: water-ethanol crude extract (7 : 3), MDM: methanol crude extract, CP: ciprofloxacin, CPC: Centre Pasteur du Cameroun. The values in bold show the most active extracts.
3.3. Antioxidant Activity of the Methanolic Crude Extract
3.3.1. Estimation of the Kinetics of the Reaction
The kinetics of reduction of the DPPH· free radical obtained are shown for each concentration of ascorbic acid in Figure 1 and in Figure 2 for the methanolic extract of D. microcarpum.
Figure 1.
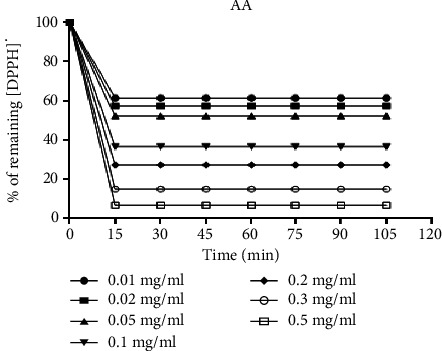
The kinetics of reduction of the DPPH· free radical with the ascorbic acid (AA).
Figure 2.

The kinetics of reduction of the DPPH· free radical with the methanolic extract of D. microcarpum (Det).
Table 3 shows the antioxidant activity of the methanolic extract of D. microcarpum.
Table 3.
Antioxidant activity of methanolic extract of D. microcarpum.
| Plants/control | Extracts/reference | DPPH· IC50 µg/mL | ABTS IC50 µg/mL | H2O2 IC50 µg/mL |
|---|---|---|---|---|
| D. microcarpum | Methanolic | 228 ± 0.04∗∗∗ | 191 ± 0.2∗∗∗ | 233 ± 0.07∗∗∗ |
|
| ||||
| Positive control | Ascorbic acid | 7.36 ± 0.31 | 19 ± 0.03 | 19.1 ± 0.02 |
| Quercetin | 21 ± 0.02 | NT | NT | |
NT: not tested; (∗∗∗) = p < 0.001.
3.4. Acute Oral Toxicity of D. microcarpum Root Bark
Table 4 shows the results of oral acute toxicity.
Table 4.
Effects of methanolic extract on liver, kidney, and haematological parameters.
| Parameters analysed | D0 | D1 | D7 | D14 |
|---|---|---|---|---|
| ALAT (UI/L) | 49.13 ± 0.14 | 47.17 ± 0.34∗∗ | 44.23 ± 01.35∗ | 41.00 ± 3.03ns |
| ASAT (UI/L) | 102.30 ± 10.42 | 101.90 ± 7.14ns | 99.40 ± 2.10∗ | 96.26 ± 6.00∗∗ |
| Uraemia (g/L) | 0.53 ± 0.01 | 0.53 ± 0.03ns | 0.58 ± 0.00∗ | 0.60 ± 0.06ns |
| Creatininaemia (mg/L) | 9 ± 2 | 10 ± 1ns | 9 ± 3ns | 8 ± 2∗∗ |
| Red blood cells (106/µL) | 9.66 ± 0.17 | 8.73 ± 0.44ns | 9.30 ± 0.55ns | 9.54 ± 0.49∗∗ |
| White blood cells (103/µL) | 13.06 ± 3.25 | 11.57 ± 2.10ns | 14.84 ± 5.31ns | 10.51 ± 4.11ns |
ALAT: alanine amino-transférase; ASAT: aspartate amino-transférase, (∗) = p < 0.033; (∗∗) = p < 0.002; ns = not significant.
4. Discussion
4.1. Extraction Yield
The extraction yield expressed as a percentage of nonvolatile compounds is shown in Table 1. Analysis of this table shows that the water-ethanol extract (34.7%) from the root bark has the highest yield, followed by the methanol extract (27.5%). The lowest yield was obtained for the dichloromethane extract (10.2%). These results are important for assay analysis to quantify total secondary metabolism levels and evaluate the various biological assays.
4.2. Anti-Shigella Activity of D. microcarpum Root Bark
Among the 4 bacterial strains tested, two were susceptibles, Shigella boydii and Shigella flexneri (Table 2). According to the criteria of Kuete in 2010 [10] (Table 5), the dichloromethane crude extract was inactive against these two strains (Shigella boydii and Shigella flexneri). The crude methanol, ethanol, and water/ethanol extracts showed the best activity with MIC at 500 µg/mL against the Shigella boydii strain. On Shigella flexneri only the methanolic extract showed moderate activity at 1000 µg/mL.
Table 5.
Scale for assessing the anti-shigella activity of extracts as a function of MIC values [10].
| Extract | Activity |
|---|---|
| CMI < 100 μg/mL | Excellent |
| 100 < CMI < 512 μg/mL | Strong |
| 512 < CMI < 2048 μg/mL | Moderate |
| CMI > 2048 μg/mL | Low |
| CMI > 10 mg/mL | Inactive |
Previous phytochemical studies of the methanolic extract of the D. microcarpum root bark revealed the presence of sterols and polyterpenes, saponins, and polyphenols such as gallic and catechic tannins, flavonoids [15], antioxidant activity [16], and anthelmintic activity [17]. Polyphenols help stop haemorrhaging and fight microbial infections. Plants rich in polyphenols are used to tighten supple tissues, as in the case of venous veins, to drain excessive secretions, as in diarrhoea, and to repair tissues damaged by burns [18].
Bioguided fractionation of this methanolic extract of D. microcarpum could make it possible to precisely isolate the compounds responsible for the anti-shigella activity. However, it would appear that this activity is due to the joint, possibly synergistic, action of all these classes of compounds.
In addition, the reference antibiotic (ciprofloxacin) showed greater anti-shigella activity than the plant extract tested. The reference antibiotics are pure molecules [19], whereas all the extracts are nonpurified mixtures of substances. Plant extract is a mixture of many types of molecules that can have synergistic effect or antagonist.
4.3. Antioxidant Activities
Concerning the kinetics of reduction of the DPPH· free radical, the reaction is biphasic, with a rapid drop in absorbance in the first few minutes, followed by a slower stage until equilibrium is reached, for the concentrations tested for the crude extract and the positive control both (Figures 1 and 2), two zones can be distinguished.
The first one is the zone of high radical trapping kinetics observed after the first 15 minutes for ascorbic acid and Det and the zone of low DPPH· radical trapping kinetics or zone of tendency towards equilibrium observed after 15 minutes for ascorbic acid and after 45 minutes for Det. The results show that the reaction between DPPH· and ascorbic acid, Det, reaches equilibrium after a short time compared with Det.
For the antioxidant activity results (Table 3), it appears that the methanolic crude extract of D. microcarpum shows good antioxidant activity with the radicals DPPH· and ABTS with IC50 at 228 and 191 µg/mL, respectively. The activity on H2O2 is lower than the others where the IC50 is at 233 µg/mL. Based on the statistical analysis, there was a significant difference between the antioxidant activity of the methanolic extract of D. microcarpum and that and ascorbic acid. This antioxidant activity could be linked to the chemical composition of this plant, and previously, some authors showed that this plant contained polyphenolic compounds, tannins, and flavonoids that are responsible for antioxidant activity [15, 16]. These results could justify the antibacterial activity of this extract.
4.4. Oral Acute Toxicity
Family self-medication is based on ancestral knowledge and the knowledge of traditional practitioners and herbalists about medicinal plants [20]. The recipes prescribed by herbalists and traditional practitioners have quality standards at all, as there are no formulations for traditional recipes from Niger. In addition, the risks of toxicity due to unfamiliarity with plants and the lack of a defined dosage and appropriate instructions for use mean that people need to be aware of the dangers of taking herbal medicines.
4.5. Behavioural Parameters
Oral administration of a single dose of 2000 mg/kg body weight of Detarium microcarpum root extract did not cause any deaths in the mice treated during the 14th day of experimentation. However, a number of changes were observed immediately after administration of the extract, such as restlessness, a decrease in mobility, and an increase in drowsiness during the first two days, but they returned to normal after the third day.
No particular signs of acute oral toxicity were observed in mice for 14 days. The LD50 was estimated to be greater than 2000 mg/kg, suggesting that Detarium microcarpum root macerate is safe to use.
4.6. Liver, Kidney, and Haematological Parameters
Transaminases or amino transferases are tissue enzymes that catalyse the transfer of alpha-amino radicals from alanine and aspartic acid to alpha-ketoglutaric acid.
ALAT transaminases are present in the liver, but also in muscle, and are more specific for liver and can help know an eventual damage of this organ [21, 22]. ALAT is a cytosolic enzyme secreted by liver cells from which it is released into the blood in the hepatic cell necrosis [23]. ASAT, found in the kidneys, pancreas, lungs, and skeletal muscle, is an indicator of hepatocyte destruction and is slightly more sensitive [21].
ALAT and ASAT levels rise rapidly when the liver is damaged for many reasons, including hepatic cell necrosis, hepatitis, cirrhosis, and the hepatotoxicity of certain drugs [22, 23].
In this study, the concentration of these two enzymes (ALAT and ASAT) was significantly reduced in animals treated with a single dose of 2000 mg/kg body weight (Table 4), suggesting that the extract has a hepatoprotective action at this dose. The statistical analysis shows that ALAT values vary significantly between D0, D1, and D7; There was a nonsignificant difference in ASAT value between D0 and D1; the difference was significant between D1, D7, and D14, suggesting that the extract has a hepatoprotective action at this dose.
The analysis of renal functions (uraemia and creatininaemia) revealed that administration of the extract did not show significant changes between D0 and D7 and the difference was significant, the D14 for creatininaemia. Serum uraemia and creatininaemia are considered the main markers of nephrotoxicity [24].
Haematological analysis (red and white blood cells) revealed no significant changes in mice treated with the single dose of 2000 mg/kg for white cells between D0 and D14, and the difference was significant, the D14 for red cells (Table 4).
4.7. Chemical Composition of D. microcarpum Extract
Considering the medicinal importance of this tree in West Africa, several phytochemical and pharmacological studies were conducted on the different organs of D. microcarpum.
Phytochemical screening showed that the methanolic extract of D. microcarpum root bark contains sterols and polyterpenes, polyphenols, flavonoids, tannins, saponosides, anthocyanins, and coumarins [15, 25].
The methanol extract of D. microcarpum roots and its fraction significantly reduced blood glucose levels in alloxan-diabetic rats without producing hypoglycemia, an effect attributed to the flavonoids abundantly present in the extract [26].
Previous studies have revealed that the methanol extract and its fractions of D. microcarpum root bark produced 100% lethality on ground glass (pheretima posthuma) at the concentration of 2 mg/mL after a time (1–3 h), compared with the results of other researchers working on other ground glass species and other plants (lethality time greater than 6 h) [27–30]. The doses of the extract used to obtain the 100% lethality time would explain the differences observed.
Chemical profiles carried out on the methanolic extract revealed the presence of flavonoids and tannins [15], while studies by Vidyadhar et al., 2010, and Deore et al., 2009, showed that flavonoids and tannins are involved in antiparasitic and antibacterial activity.
Rhinocerotinoic acid has been isolated from root barks and shows activities against Salmonella typhi and Salmonella enteritidis [31].
5. Conclusion
This work shows that ethanolic, methanolic, and hydroethanolic extracts of D. microcarpum root bark are the best active against the shigella flexneri strain. This result demonstrates the anti-shigella potential of root barks and contributes to the justification of using this plant in the treatment of gastrointestinal disorders. The methanolic crude extract of D. microcarpum has a good antioxidant activity. The results of in vivo oral toxicity tests on mice showed no acute toxicity for a dose less than or equal to 2000 mg/kg for 14 days. The LD50 was estimated to be greater than 2000 mg/kg, which suggests that D. microcarpum root macerate is safe to use. Further studies are needed to perform a bioguided isolation of active compounds against shigella.
Acknowledgments
The authors would like to acknowledge the Yaoundé-Bielefeld Graduate School of Natural Products with Antiparasitic and Antibacterial Activities (YaBiNaPA, project no. 57316173).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Drouet P. E. The microbial word: part 1. 2012.
- 2.Who. WHO Strategy for Traditional Medecine for 2002–2005 . Genève, Switzerland: WHO; 2002. [Google Scholar]
- 3.Stephen J. Pathogenesis of infectious diarrhea. Canadian Journal of Gastroenterology . 2001;15(10):669–683. doi: 10.1155/2001/264096. [DOI] [PubMed] [Google Scholar]
- 4.Maman I., Diallo B. A., Ousmane S., Salaou C., Testa J. Acute diarrheas caused by salmonella and shigella in under five years children at the Niamey national hospital (Niger) International Journal of Mechanical Engineering Education . 2018;2(3):96–103. [Google Scholar]
- 5.Mwambete K. D. The in vitro antimicrobial activity of fruit and leaf crude extracts of Momordica charantia: a Tanzania medicinal plant. African Health Sciences . 2009;9(1):34–39. [PMC free article] [PubMed] [Google Scholar]
- 6.Khalid I., Garba M., Saadou M. Research on Traditional Pharmacopoeia in Niger . Niamey, Niger: CELHTO; 1984. [Google Scholar]
- 7.Adjanohoun E. J., Adjakidjè V., Ahyi M. R. A., Aké Assi L., Akoègninou A. Traditional Medicine and Pharmacopoeia: Contribution to Ethnobotanical and floristic Studies in Niger . 1989: Agency for Cultural and Technical Cooperation; Paris, France. [Google Scholar]
- 8.Clsi. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard-Ninth Edition. CLSI Document M07-A9 . Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2012. [Google Scholar]
- 9.Abdoulahi M. I. I., Yanick Kevin M. D., Lauve Rachel T. Y., et al. Antibacterial activity of eight medicinal plants from the traditional pharmacopoeia of Niger. Journal of Tropical Medicine . 2023;2023:10. doi: 10.1155/2023/6120255.6120255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuete V. Potential of Cameroonian plants and derived products against microbial infections: a review. Planta Medica . 2010;76(14):1479–1491. doi: 10.1055/s-0030-1250027. [DOI] [PubMed] [Google Scholar]
- 11.Brand-Williams W., Cuvelier M. E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Science and Technology . 1995;28(1):25–30. doi: 10.1016/s0023-6438(95)80008-5. [DOI] [Google Scholar]
- 12.Llorach R., Tomás-Barberán F. A., Ferreres F. Lettuce and chicory byproducts as a source of antioxidant phenolic extracts. Journal of Agricultural and Food Chemistry . 2004;52(16):5109–5116. doi: 10.1021/jf040055a. [DOI] [PubMed] [Google Scholar]
- 13.Wang S. Y., Jiao H. Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals and singlet oxygen. Journal of Agricultural and Food Chemistry . 2000;48(11):5677–5684. doi: 10.1021/jf000766i. [DOI] [PubMed] [Google Scholar]
- 14.OCDE. Ligne directrice de l’OCDE pour les essais de produits chimiques 407. 2008.
- 15.Habibou H. H., Moutari S. K., Lawaly M. M., Idrissa M., Rabani A., Khalid I. Phytochemical screening and determination of polyphenols in Detarium microcarpum (Guill. and Perr.) used in the treatment of parasitic diseases in Niger. Afrique Science . 2018;14(5):390–399. [Google Scholar]
- 16.Habibou H. H., Idrissa M., Khalid I., Benjamin O., Rabani A. Antioxidant activity of methanolic extracts from various plant organs of Detarium microcarpum Guill. and Perr. European Scientific Journal ESJ . 2019;15(12):159–171. doi: 10.19044/esj.2019.v15n12p159. [DOI] [Google Scholar]
- 17.Habibou H. H., Chaibou M., Zakari C. O., Rabani A., Khalid I. Phytochemical study and anthelmintic activity of fractions of the root extract from Detarium microcarpum Guill. and Perr. Revue Francophone Internationale . 2019;27(2) [Google Scholar]
- 18.Andrew C. Encyclopedia of Medicinal Plants . 2nd 2001. [Google Scholar]
- 19.Sourabie T. S., Nikiema J., Lega I., Nacoulma O. G., Guissou I. P. Etude in vitro de l’activité antibactérienne d’extraits d’une plante de la pharmacopée burkinabé: cas d’Argemone mexicana L. (Papaveraceae) International Journal of Brain and Cognitive Sciences . 2011;4(6):2009–2016. doi: 10.4314/ijbcs.v4i6.64954. [DOI] [Google Scholar]
- 20.Amina B. Phytochemical Screening, Toxicological Study and Pharmacological Valorization of Melissa officinalis and Mentha rotundifolia (Lamiaceae) Rabat, Morocco: Mohamed V University of Morocco; 2016. [Google Scholar]
- 21.Goddard C., Warnes T. Raised liver enzymes in asymptomatic patients: investigation and outcome. Digestive Diseases . 1992;10(4):218–226. doi: 10.1159/000171360. [DOI] [PubMed] [Google Scholar]
- 22.Pratt D. S., Kaplan M. M. Evaluation of abnormal liver-enzyme results in asymptomatic patients. New England Journal of Medicine . 2000;342(17):1266–1271. doi: 10.1056/nejm200004273421707. [DOI] [PubMed] [Google Scholar]
- 23.Dufour D. R., Lott J. A., Nolte F. S., Gretch D. R., Koff R. S., Seeff L. B. Diagnosis and monitoring of hepatic injury. II. Recommendations for use of laboratory tests in screening, diagnosis, and monitoring. Clinical Chemistry . 2000;46(12):2050–2068. doi: 10.1093/clinchem/46.12.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palani S., Raja R., Kumar P., Jayakumar S. Therapeutic efficacy of Pimpinella tirupatiensis (Apiaceae) on acetaminophen induced nephrotoxicity and oxidative stress in male albino rats. International Journal of Pharmacy and Technology . 2009;2:708–719. [Google Scholar]
- 25.Loubaki B. C., Ouattara A. S., Ouattara C. A., Ouedraogo T. R., Traore A. S. Activités antimicrobiennes des extraits aqueux totaux de Detarium microcarpum [Cesalpinaceae (Gull. et Perr.)] sur huit espèces bactériennes impliquées dans certaines maladies infectieuses au Burkina Faso. 1991.
- 26.Okolo C. E., Akah P. A., Uzodinma S. U. Antidiabetic activity of root extract of Detarium microcarpum Guill. and Perr. Phytopharmacology . 2012;3(1):12–18. [Google Scholar]
- 27.Guissou L. P., Ouedraogo S., Sanfo A., Some N., Lompo M. Mise au point d’un modèle biologique de test antiparasitaire appliqué aux plantes médecinales. Pharm. Traditional African Medicine . 1988;10:105–133. [Google Scholar]
- 28.Vidyadhar S., Saidulu M., Gopal T. K., Chamundeeswari D., Umamaheswara R., Banji D. In vitro anthelmintic activity of the whole plant of Enicostemma littorale by using various extracts. International Journal of Applied Biology and Pharmaceutical Technology . 2010;1(3):1119–1125. [Google Scholar]
- 29.Satish B. K., Ravindra A. F. Investigation of in vitro anthelmintic activity of Thespesia lampas. Asian Journal of Pharmaceutical and Clinical Research . 2009;2(2):69–71. [Google Scholar]
- 30.Kumar A. B., Lakshman K., Jayaveera K. N., Nandeesh R., Manoj B., Ranganayakulu D. Comparative in vitro anthelmintic activity of three plants from the Amaranthaceae family. Archives of Biological Sciences . 2010;62(1):185–189. doi: 10.2298/abs1001185k. [DOI] [Google Scholar]
- 31.Mbock M. A., Fouatio W. F., Kamkumo R. G., et al. In vitro and in vivo anti-salmonella properties of hydroethanolic extract of Detarium microcarpum Guill. and Perr. (Leguminosae) root bark and LC-MS-based phytochemical analysis. Journal of Ethnopharmacology . 2020;260 doi: 10.1016/j.jep.2020.113049.113049 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


