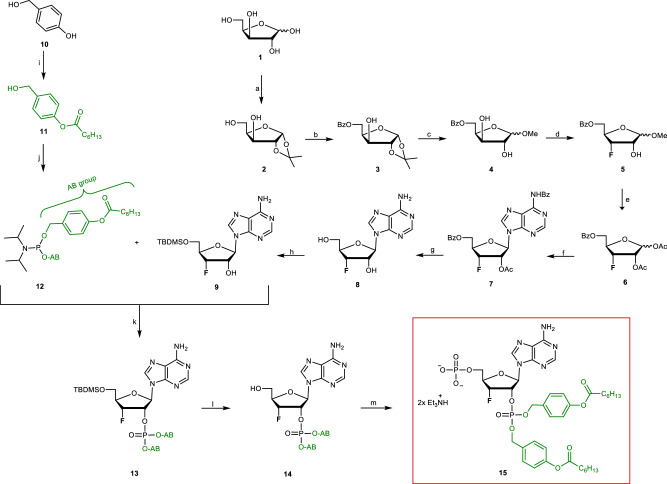
Fig. 1. Synthesis of MASTER-NAADP and derivatives I Reagents and conditions.
[a] (i) 0.10 eq. CuSO4, 2.8 eq. conc. H2SO4, acetone, 3 h, rt, (ii) HCl-solution (0.2 %), 6 h, rt, 99 %; [b] 1.5 eq. Et3N, 1.1 eq. BzCl, CH2Cl2, 1 h, 0 °C, 92 %; [c] 0.2 eq. I2, MeOH, 4 h, 70 °C, 86 %; [d] 1.5 eq. DAST, dry CH2Cl2, 20 h, -78 °C → rt, 43 %; [e] 4.7 eq. Ac2O, 4.7 eq. conc. H2SO4, AcOH, 19 h, rt, 93 %; [f] (i) 1.5 eq. N-benzoyladenine, 1.2 eq. BSA, C2H4Cl2, 1 h, 90 °C, (ii) 1.0 eq. 6, 4.0 eq. TMSOTf, C2H4Cl2, 5.5 h, 90 °C, 61 %; [g] ammonia 7 N in MeOH, 28 h, 70 °C, 84 %; [h] 1.5 eq. TBDMSCl, dry pyridine, 24 h, rt, 71 %; [i] 1.2 eq. 4-hydroxyl benzyl alcohol 10, 1.2 eq. Et3N, 1.0 eq. heptanoyl chloride, THF, 2 h, 0 °C, 87 %; [j] 1.0 eq. dichloro-N,N-diisopropylaminophosphoramidite, 2.2 eq. 11, 2.3 eq. Et3N, THF, 0 °C → rt, 16 h, 92 %; [k] (i) 1.3 eq. 12, 1.2 eq. pyridinium trifluoracetate, CH2Cl2, 50 min, rt, (ii) 1.5 eq. tert-butylhydroxyperoxide (5.0 M in decane), 0 °C, 1 h, 87 %; [l] 5.2 eq. triethylamine-trihydrofluoride, CH2Cl2, 19 h, rt, 81 %; [m] 4.0 eq. POCl3, TMP, 6.5 h, 0 °C, 31 %.