Abstract
Background
Memantine, which is an FDA-proven drug for the treatment of dementia, exerts its function by blocking the function of NMDA (N-methyl-D-aspartate) receptor, a calcium-permeable ion channel that reduces cytotoxic calcium overload. Chondrocyte senescence is a crucial cellular event that contributes to articular cartilage degeneration during osteoarthritis (OA) development. To date, the effects of memantine and its downstream NMDA receptor on chondrocyte senescence and OA have been rarely reported.
Methods
The protein levels of NMDA receptor and its agonistic ligand, glutamate, were compared between normal and OA chondrocytes. The quantity of intracellular calcium ions and the level of mitochondrial damage were evaluated using specific fluorescent probes and transmission electron microscopy (TEM), respectively. Chondrocyte senescence was evaluated by senescence-associated β-galactosidase (SA-β-Gal) staining and p16INK4a analysis. The function of NMDA receptor in chondrocyte senescence and OA was tested via agonists activation and gene knockdown experiments. The therapeutic effects of memantine on OA were examined both in vitro and in vivo. Additionally, to verify the findings from animal samples, a propensity score-matched cohort study was conducted using data from a United Kingdom primary care database (i.e., IQVIA Medical Research Database [IMRD]) to compare the risk of OA-related joint replacement involved in memantine initiators versus active comparators (i.e., acetylcholinesterase [AchE] initiators) in patients with dementia.
Results
The protein expression of NMDA receptor and the secretion of glutamate were both significantly increased in OA chondrocytes. NMDA receptor activation was found to stimulate chondrocyte calcium overload, which further led to mitochondrial fragmentation and chondrocyte senescence. Blocking the NMDA receptor with memantine and N-methyl-D-aspartate receptor subunit 1(NR1, the gene encoding NMDA receptor) knockdown resulted in reduced calcium influx, mitochondrial fragmentation, as well as cellular senescence in OA chondrocytes. Intra-articular injection of memantine in OA mice also exhibited protective effects against cartilage degeneration. Moreover, in the 1:5 propensity score-matched cohort study consisting of 6218 patients (n = 1435 in the memantine cohort; n = 4783 in the AchE cohort), the memantine initiator was associated with a lower risk of OA-related joint replacement than AchE initiators (Hazard ratio = 0.56, 95 % confidence interval: 0.34 to 0.99).
Conclusion
NMDA receptor plays an important role in inflammatory-induced cytotoxic calcium overload in chondrocytes, while memantine can effectively block the NMDA receptor to reduce chondrocyte senescence and retard the development of OA.
The translational potential of this article
As a clinically licensed drug used for the treatment of dementia, memantine has shown promising therapeutic effects on OA. Mechanistically, it functions by blocking NMDA receptor from mediating chondrocyte senescence. The protective effects of memantine against OA were verified not only by in vivo and in vitro experiments but also via a propensity score-matched human cohort study. These findings presented robust evidence for repurposing memantine for the treatment of OA.
Keywords: Chondrocyte senescence, Memantine, Mitochondria, NMDA receptor, Osteoarthritis
Graphical abstract
1. Introduction
Osteoarthritis (OA) is the most common age-related joint disease and the leading cause of disability in elderly adults. Unfortunately, there is currently a lack of effective therapeutic approaches to cure this disease due to the fact that the underlying mechanism of OA is still unclarified. Cartilage degradation is considered the major hallmark of OA [1]. As the only cell type in articular cartilage, homeostasis between the catabolic and anabolic metabolism of chondrocytes is crucial for cartilage health, and chondrocyte senescence has emerged to be one of the core cellular events that can disturb such homeostasis. Senescent chondrocytes not only present cellular functional disorder but also exhibit a senescence-associated secretory phenotype (SASP) that contains cartilage-digesting enzymes and inflammatory cytokines, which can result in cartilage degradation [2]. Recently, it was reported that senescent chondrocytes were significantly increased in human OA degenerative cartilage compared with that in preserved cartilage [3], suggesting a strong correlation between chondrocyte senescence and OA severity [4]. A growing body of evidence has shown that targeting senescent chondrocytes in the mouse joint can attenuate OA progression [5]. Thus, revealing the causes of chondrocyte senescence and seeking targeted drugs is an important research direction with potential therapeutic application value.
Memantine is an FDA-approved medication for the treatment of neurodegenerative disorders such as Alzheimer's disease [6]. An increasing number of studies have shown that memantine also possesses the potential to be used in treating intracerebral hemorrhage, brain metastases, inflammatory disease, etc., implying a broad prospect of repurposing memantine in many other clinical scenarios. Memantine, as an N-methyl-d-aspartate (NMDA) receptor modulator/agonist, primarily exerts its function by blocking the NMDA receptor and eliciting the excessive influx of calcium ions [7]. Mitochondria are the main organelle for intracellular calcium homeostasis. Excessive calcium ions in the cytoplasm will be transported to the inner mitochondrial membrane by the calcium uniporter, which can disrupt the mitochondrial membrane potential, ultimately leading to mitochondrial dysfunction and cellular senescence [8]. Previous research has revealed that calcium overload can significantly induce the expression of matrix metalloproteinases (MMPs) and inflammatory cytokines in chondrocytes [9]. Meanwhile, articular chondrocytes were also reported to express NMDA receptor [10]. However, the role of NMDA receptor in chondrocyte calcium overload still warrants elucidation, and evidence regarding the effects of memantine on OA needs to be further strengthened [11].
In this study, we found that the expression level of NMDA receptor correlates with the severity of OA cartilage degradation. Inflammatory cytokines and NMDA receptor agonists can induce calcium overload, mitochondrial fragmentation, as well as chondrocyte senescence, while NMDA receptor knockdown or memantine use can inhibit such effects and eventually enhance the anabolic metabolism of chondrocytes. Moreover, the chondroprotective action of memantine was further verified in surgically-induced OA mice and via a population-based cohort study. Overall, our findings present new evidence for repurposing memantine for the treatment of OA.
2. Materials and methods
2.1. Reagents and antibodies
The memantine and glutamate were purchased from Sigma–Aldrich (Saint Louis, USA). The N-Methyl-D-aspartic acid (NMDA) was purchased from Selleck (Shanghai, China). The recombinant mouse tumor necrosis factor-α (TNF-α) protein was obtained from Peprotech (Shanghai, China). Collagenase Type Ⅱ was obtained from Bioforxx (Beijing, China). The fetal bovine serum (FBS), Dulbecco's Modified Eagle Medium (DMEM/F12), and Lipofectamine RNAiMax Transfection Reagent were purchased from Thermo Fisher Scientific (Dreieich, Germany). Small interfering RNAs (siRNAs; specifically, siNC: SC-36869 and siNR1: SC-36082) were obtained from Santa Cruz Biotechnologies (CA, USA). The Senescence-Associated β-Galactosidase (SA-β-Gal) Staining kit, Hoechst 33342 staining kit, Fluo-4 AM fluorescence probe, Mito-Tracker Red CMXRos fluorescence probe and JC-1 fluorescence staining kit were obtained from Beyotime (Shanghai, China). Besides, the following antibodies were used in this study: anti-Collagen Ⅱ (28459-1-AP) and anti-MMP13 (18165-1-AP) from Proteintech Group (Wuhan, China); anti-NMDAR1 (ab193310) from Abcam (Cambridge, USA); anti-P16INK4a (sc-1661) and anti-GAPDH (sc-32233) from Santa Cruz Biotechnologies (CA, USA).
2.2. Culture of primary articular chondrocytes
With approval from the ethics committee of Xiangya Hospital, Central South University (Approval number: 202110186), human OA cartilages were obtained from patients who underwent total knee arthroplasty (n = 3; aged 66 ± 3.03 years). Preserved and damaged cartilages were first distinguished by Outerbridge scoring and then harvested. To obtain primary human chondrocytes, the cartilage was cut into pieces and incubated with pronase (2 mg/mL) in DMEM/F12 without serum at 37 °C for 1 h, and further digested with collagenase P (3.6 mg/mL) in DMEM/F12 with 5 % serum at 37 °C overnight.
The primary murine articular chondrocytes were extracted following our previous protocol [12]. Briefly, the cartilage tissue was collected from postnatal day 3–4 C57BL/6 mice, and the cartilage samples were then incubated in 0.1 % collagenase 2 solution overnight. The chondrocytes were cultured in DMEM/F12 medium (Gibco, USA) containing 10 % FBS (Gibco, USA) supplemented with 1 % penicillin/streptomycin antibiotics (Gibco, USA).
2.3. Chondrocytes treatment and siRNAs transfection
The primary murine chondrocytes were cultured in the presence of 10 ng/mL TNF-α to mimic OA circumstance. Two agonists of the NMDA receptor, namely glutamate (500 μM) and NMDA (100 μM), were used to treat the chondrocytes to activate the NMDA receptor in vitro. Then, the chondrocytes were cultured in the medium with memantine (10 μM) and TNF-α (10 ng/mL) and in the medium with TNF-α (10 ng/mL) alone, respectively. A control sample of chondrocytes cultured without memantine and TNF-α was also evaluated for comparison purpose.
The siRNAs targeting NR1 (si-NR1), i.e., the gene encoding NMDA receptor, was used in this study, with a scrambled siRNA (si-NC) as the negative control. Briefly, the chondrocytes at 50–60 % confluence were transfected with siRNAs using the Lipofectamine RNAiMAX reagent as described previously [3]. After 12 h of incubation, the transfection medium was replaced with growth medium. The transfected cells were then used for other analyses.
2.4. Senescence-associated β galactosidase staining (SA-β-gal staining)
Chondrocyte senescence was evaluated using a SA-β-gal staining kit (Beyotime, China) according to the manufacturer's instructions. The percentage of SA-β-gal-positive chondrocytes was calculated and analyzed in randomly selected fields from three repeated experiments under an optical microscope (Nikon, Japan).
2.5. JC-1 staining
To measure the mitochondrial membrane potential, we utilized the JC-1 staining kit (Beyotime, China) to label with hochest 33342 (Beyotime, China) in the dark at 37 °C for 20 min. The images were acquired with laser scanning confocal microscopy (CSU-W1, Nikon, Japan). The red fluorescence intensity (excitation 525 and emission 590) represented JC-1 aggregates, while the green fluorescence intensity (excitation 90 and emission 530) represented JC-1 monomers. The red fluorescence and green fluorescence were analyzed using the semi-automated morphometric tool Image J 1.53 t software.
2.6. Measurement of intracellular Ca2+
The chondrocytes were incubated in a 2 μM Fluo-4 AM Ca2+ fluorescent probe (Beyotime, China) for 30 min at 37 °C and 5 % CO2. Then, the fluorescence from Fluo-4 AM (excitation 485 nm, emission 525 nm) was recorded within 5 min under a fluorescence microscope (Zeiss, Germany).
2.7. Analysis of mitochondrial morphology
To determine mitochondrial fragmentation in the chondrocytes, the Mito-Tracker Red chloromethyl-X-rosamine (CMXRos) staining kit (Beyotime, China) was used to label mitochondria, at an excitation of 579 nm and an emission of 599 nm, according to the manufacturer's instructions. The images were captured using a confocal microscopy (Zeiss, Germany), and the mitochondrial morphology was evaluated and analyzed using the semi-automated morphometric tool MiNA of Fiji software.
2.8. Transmission electron microscopy (TEM)
The mouse chondrocytes were fixed in 2.5 % glutaraldehyde overnight, and further fixed in 2 % osmium tetroxide for 1 h and stained with 2 % uranyl acetate for 1 h. After dehydration in a series of acetone, the samples were embedded in araldite and cut into semi-thin sections, which were stained with toluidine blue to locate cell position for observation under TEM (Hitachi, Tokyo, Japan). Two pathologists were engaged to evaluate the mitochondrial morphology of each sample independently and blindly, and the mitochondrial number, perimeter and area of each sample were counted as described previously [13].
2.9. Immunostaining
For immunofluorescence staining (IF), the chondrocytes were rinsed with PBS first, and fixed in 4 % paraformaldehyde and permeated in 0.1 % Triton X-100 for 10 min. Then, the chondrocytes were blocked with 5 % bovine serum albumin for 1 h at 37 °C, and were further rinsed with PBS and incubated with primary antibodies in a humid chamber at 4 °C overnight. Subsequently, the chondrocytes were rinsed and incubated with the Alexa Fluo-488 anti-rabbit or Alexa Fluor 594 anti-mouse conjugated second antibodies at room temperature for 1 h, and were labeled with DAPI for 5 min. The fluorescence intensity was measured using Image J software 2.1 (Bethesda, USA).
For immunohistochemistry (IHC), the rehydrated slices were treated using an immunohistochemical kit (Zhongshan Jinqiao, China), and the primary antibodies, i.e., MMP13 (1:200), COL2A1 (1:200) and P16INK4a (1:100), were used for investigation. On the next day, the slices were rinsed with PBS and incubated in the biotinylated secondary antibody for 1 h. Then, the slices were further incubated with horseradish peroxidase-conjugated streptavidin for 0.5 h and visualized by the Vector NovaRED™ peroxidase substrate. Thereafter, counterstaining was conducted using Hematoxylin (Vector Labs, USA), and semi-quantification was conducted using Pro Plus 6.0 software (Media Cybernetics, USA).
2.10. Animal experiment
All animal experiments were approved by the medical ethical committee of Xiangya Hospital of Central South University (2022020438, Changsha, China), and were performed in compliance with the Guidelines for Care and Use of Laboratory Animals of the National Institutes of Health. To establish mouse OA models, the destabilization of medial meniscus (DMM) surgery was performed on C57BL/6 mice [14]. Briefly, the DMM model was created via transection of anterior medial meniscotibial ligament using a microsurgical knife. Complete disruption of ligament was visually confirmed by manually displacing the medial meniscus with fine forceps. Then, sham surgery, which consists of skin incision and medial capsulotomy only, was performed on the right knees of a separate group of mice, followed by capsule and skin closure as described above. Another group of DMM mice were injected with 100 μM Mem from 3 days after the DMM surgery following a protocol of twice per week, 10 μL per joint [15,16]. In order to investigate the analgesic effects of memantine, we further adopted an OA-related joint pain model [17,18]. Male Sprague Dawley rats (Charles River, UK) weighting 180–220 g were used. Rats were briefly anaesthetised with isoflurane. Joint damage was induced by a single intra-articular injection of monosodium iodoacetate (MIA, 2 mg/40 μL; Sigma, UK) of the right knee. Control animals received a single injection of saline (40 μL) into the right knee. Three separate measures of pain behaviour were assessed: change in hind limb weight distribution, hot plate behavior and hind paw mechanical withdrawal thresholds, which provide a comprehensive monitoring of pain assessment.
2.11. Von Frey test
We employed the electronic von Frey test (IITC, USA) to assess the response to mechanical stimulation on the right hind paws of all rats on days 0, 3, 7, 10, 14, 17, 21, 25, and 28 [19]. Before testing, the rats were placed in an organic glass chamber (8 cm × 16 cm × 9 cm) for 30 min. After zeroing the instrument, pressure was applied to the rat's paws from the bottom using a 0.8 mm fiber-tipped filament. When the rat lifts its paw, it indicates that it has sensed the applied pressure, and the maximum force applied to the tip of the device was automatically recorded through a manageable force transducer, with the paw withdrawal threshold displayed on the screen. The force needed to retract the hind paw was recorded three times at 5-min stimulation intervals. Von Frey test was performed during the light cycle by the same researcher who was blinded to the treatments.
2.12. Hot plate test
The Hargreaves method was used to measure the thermal pain threshold of the hind paw using the Plantar Test (Ugo Basile, Italy). Before testing, untrained animals were domesticated in acrylic compartments (8 cm × 16 cm × 9 cm) on a uniform glass surface for up to 30 min. Guide the infrared light source onto the surface of the right paw of all rats on days 0, 3, 7, 10, 14, 17, 21, 25, and 28, maintaining a temperature of 52 °C, and immediately remove any paw pads after licking them, while recording the time spent on the hot plate (reaction time). Record each hind paw retraction during a test with a minimum 5-min interval between each stimulus. To avoid tissue damage, the maximum allowed stimulation latency is 30 s. Hot plate test was performed during the light cycle by the same researcher who was blinded to the treatments.
2.13. Weight-bearing measurement
Weight-bearing was evaluated using an incapacitance tester (IITC, USA) that included a dual-channel weight mean value [19]. After zeroing the instrument, the rats were attentively positioned in a plastic chamber. The force applied by an individual hind limb was averaged over more than a 5-s time. The individual data point was the average of 3 measurements. The percentage of weight borne by the handled (ipsilateral) hind limb was calculated utilizing the following equation: (Weight on Right Leg/Weight on Right Leg and Left Leg) × 100. A value of 50 % represents an equal weight distribution across ipsilateral and contralateral hindlimbs, while a value of less than 50 % suggests a reduction in weight borne on the ipsilateral hind limb. Rats were assessed on days 0, 3, 7, 10, 14, 17, 21, 25 and 28 after injection. Each session involved three distinct trials with a minimum 5-min interval between them. Weight-bearing measurement was performed during the light cycle by the same researcher who was blinded to the treatments.
2.14. Histology (safranin O/fast green staining)
The paraffin-embedded mouse knee joint samples were sectioned at a thickness of 5 μm, and safranin O/fast green staining was then performed as previously described [20]. To evaluate the severity of cartilage degradation, three experienced researchers were engaged to review and score the slices independently and blindly based on a semi-quantitative histopathological scoring system recommended by the Osteoarthritis Research Society International (OARSI) on a scale of 0–6.
2.15. Population-based cohort study
2.15.1. Data source
We used data from IQVIA Medical Research Database (IMRD), incorporating data from The Health Improvement Network (THIN), a Cegedim database from general practitioners (GPs) in the United Kingdom, as the basis of investigation in this study. The IMRD contains anonymized medical records from 839 general practices, covering approximately 19 million patients. For the THIN database, a previous study has demonstrated its validity in clinical and epidemiological research. The scientific review committee for IMRD and the institutional review board at Xiangya Hospital approved this study with a waiver of informed consent (21SRC056, 2018091077).
2.15.2. Study design and cohort definition
A 1: 5 propensity score (PS)-matched cohort study was conducted to compare the risk of joint replacement between patients initiating memantine and those initiating active comparators (acetylcholinesterase [AChE]). All the participants included in this study were aged 40–90 years old and diagnosed with OA and dementia, and had at least one year of active enrolment with general practice from January 2000 to December 2019. The initiators of memantine or AChE were identified based on the first record of prescription after the diagnosis of both OA and dementia. The date of the first prescription of either memantine or AChE was regarded as the index date. Subjects were excluded if they had been prescribed with comparators during the 1-year timeframe before the index date, or had a history of joint replacement before the index date, or had missing information on body mass index (BMI), smoking status, drinking status, or Townsend deprivation index (i.e., socioeconomic deprivation index) before the index date.
2.15.3. Evaluation of outcome and covariates
The outcome of this study was incident joint replacement during 5-year follow-up. Joint replacement was identified by Read codes [21].
The covariates of socio-demographic and anthropometric characteristics (i.e., age, sex, BMI, and socioeconomic deprivation index) and lifestyle factors (i.e., smoking status and alcohol use) were evaluated using the nearest available data prior to the index date. Comorbidities and medication use were evaluated at any time before the index date. Healthcare utilization (i.e., number of hospitalizations, general practice visits and specialist referrals) was evaluated during the 1-year timeframe before the index date.
2.16. Statistical analysis
For the population-based cohort study, the person-years of follow-up for each subject were calculated as the timeframe from the index date to the earliest occurrence of the followings: incident joint replacement, disenrollment from a GP practice, age of 90 years, death, end of the study (December 31,2019), or end of 5-year follow-up. The rate of incident joint replacement was calculated for each cohort, and the cumulative incidence curves of joint replacement were plotted accordingly. The rate difference (RD) and its 95 % confidence interval (CI) between the two comparison groups were also calculated. Moreover, a Cox proportional hazard model was performed to obtain the hazard ratio (HR) and its 95 % CI of the joint replacement accounting for the competing risk of death.
Other statistical analyses were performed using Prism 10 (GraphPad Software, USA). Quantitative results were presented as mean ± Standard Error for the Sample Mean (SEM). Student's t test and one-way or two-way analysis of variance (ANOVA) were used for comparison between two and multiple groups, respectively. Statistical significance was set at p < 0.05. Other statistical details, including the value and definition of n, error bars and significance thresholds, can be found in Figure Legends.
3. Results
3.1. NMDA receptor is upregulated and activated in OA chondrocytes
The expression level of NMDA receptor was first compared between normal and OA chondrocytes using samples from both mice and human donors. As shown in Fig. 1A and B, the articular chondrocytes from the DMM mouse knee exhibited a significantly higher protein level of NMDA receptor. Similar results were observed in human samples: the damaged cartilage from human donors also showed an elevated NMDA receptor level compared with the preserved cartilage (Fig. 1C and D). Moreover, an in vitro OA-like chondrocyte model was established using TNF-α stimulated murine primary chondrocytes. It was confirmed that the inflammatory environment of OA can not only elevate the protein level of NMDA receptor but also stimulate the secretion of its agonist glutamate, leading to increased calcium ion influx (Fig. 1E–H). Consequently, the level of mitochondrial fragmentation and mitochondrial membrane potential, as well as chondrocyte senescence were all elevated in TNF-α stimulated chondrocytes group (Fig. 1I–N).
Figure 1.
OA progression accompanied by highly expressed and activated levels of NMDA receptor that led to mitochondrial fragmentation and chondrocyte senescence. (A–B) Immunostaining and semi-quantification of the expression levels of NMDA receptor (Red) and Col2a1(Green) in destabilization of the medial meniscus (DMM) surgery-induced mouse OA knee joint; Blue = nucleus, scale bars = 100 μm, n = 5, data are presented as mean ± SEM and Student's t test was used (*p < 0.05). (C–D) The expression level of NMDA receptor in preserved and damaged cartilages from OA patients as revealed by Western blotting and analysis. Data are presented as mean ± SEM and Student's t test was used (N = 3, **p < 0.01). (E) The secretion of glutamate from TNF-α stimulated primary mouse chondrocytes. Data are presented as mean ± SEM and Student's t test was used (N = 3, **p < 0.01). (F–H) Immunostaining and semi-quantification of the NMDA receptor level (N = 6, scale bars = 100 μm) and calcium level (N = 4, scale bars = 10 μm) in TNF-α stimulated primary mouse chondrocytes. Data are presented as mean ± SEM and Student's t test was used (**p < 0.01). (I) Representative confocal images of mitochondrial morphology by mito-tracker (scale bar = 10 μm, the white boxed regions are further enlarged) and mitochondrial membrane potential by JC-1 (scale bar = 100 μm), TNF-α stimulated primary mouse chondrocytes were used (N = 3). (J–K) Semi-quantified measurements of mitochondrial number per cell and mean branches per network in control and TNF-α stimulated primary mouse chondrocytes. Data are presented as mean ± SEM and Student's t test was used (N = 3, **p < 0.01). (L) Semi-quantification of mitochondrial membrane potential detected by JC‐1 fluorescent probe. The ratio of JC-1 aggregates (red) and JC‐1 monomer (green) was calculated by Image J. Data were presented as the mean ± SEM. Statistical analysis was performed using Student's t test (N = 3, **p < 0.01). (M and N) The SA-β-Gal staining (scale bar = 100 μm) in control and TNF-α stimulated primary mouse chondrocytes; The ratio of SA-β-Gal positive chondrocytes were analyzed, data are presented as mean ± SEM and Student's t test was performed (N = 3, **p < 0.01).
3.2. NMDA receptor activation induces calcium overload and mitochondrial dysfunction, and further promotes chondrocyte senescence and OA phenotypes
Two agonists of the NMDA receptor, namely glutamate and NMDA, were used to examine the role of NMDA receptor in OA, and a large number of calcium ions were observed in the chondrocytes cultured with both agonists (Fig. 2A). Mitochondria are usually long and tubular in shape, with branched or unbranched cristae, acting as the major organelle that is susceptible to calcium overload. We then observed the mitochondrial morphology and membrane potential under confocal microscope and found severe mitochondrial fragmentation and low membrane potential in agonist-treated groups (Fig. 2B and C). Thereafter, the generation of senescent phenotype after agonists treatment was confirmed by SA-β-gal positive staining (Fig. 2D). Additionally, the protein levels of type II collagen (COL2A1) and matrix metalloproteinase 13 (MMP13) were detected in each group to reflect the OA-related phenotypic changes. In response to glutamate/NMDA activation, downregulation of COL2A1 (Fig. 2F) and upregulation of MMP13 (Fig. 2E) were both identified in glutamate/NMDA-treated groups.
Figure 2.
NMDA receptor agonists induce calcium overload and mitochondrial fragmentation, and further promote chondrocyte senescence and OA-like phenotypes. (A) The Fluo-4 AM probe was used to determine the calcium level induced by NMDA and Glu treated primary mouse chondrocytes (Scale bar = 100 μm). Data are presented as mean ± SEM, statistical analysis was performed using one-way ANOVA/post-hoc Bonferroni test (N = 9–17, **p < 0.01). (B) Representative confocal images of the chondrocytes mitochondria revealed by mito-tracker immunofluorescent staining (Scale bar = 5 μm). Semi-quantification of mitochondrial branches was conducted through Image J software; one-way ANOVA/post-hoc Bonferroni test was used to compare the difference between groups, data are presented as mean ± SEM (N = 5, *p < 0.05). (C) Mitochondrial membrane potential was detected by JC‐1 staining (Scale bar = 100 μm). The ratio of JC-1 aggregates (red) and JC‐1 monomer (green) was calculated by Image J. Data were presented as the mean ± SEM. Statistical analysis was performed using one-way ANOVA/post-hoc Bonferroni test (N = 3, *p < 0.05, **p < 0.01). (D) The SA-β-Gal staining (Scale bar = 100 μm) was used to determine senescent chondrocytes. SA-β-Gal staining positive cells were semi-quantified by Image J. Data are presented as mean ± SEM, and one-way ANOVA/post-hoc Bonferroni test was used (N = 5, *p < 0.05). (E–F) Immunofluorescent staining and semi-quantitative analysis of MMP13 and COL2A1 in primary mouse chondrocytes stimulated by NMDA and Glu (Scale bar = 100 μm). Data are presented as mean ± SEM and statistical analysis was performed using one-way ANOVA/post-hoc Bonferroni test (N = 5, *p < 0.05; **p < 0.01). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In summary, above results revealed that NMDA receptor agonists (NMDA and glutamate) can promote chondrocyte senescence and ECM degradation, and such effects in OA may be exerted partially via induction of calcium overload and mitochondrial dysfunction.
3.3. NMDA receptor knockdown reduces chondrocyte senescence and OA-like phenotypes by alleviating calcium overload and mitochondrial damage
By exposing chondrocytes to TNF-α treatment, with or without concomitant knockdown of NMDA receptor, we further verified the role of NMDA receptor in OA. Specifically, siRNAs were used to knockdown the NMDA receptor in both primary normal mouse chondrocytes and TNF-α stimulated mouse chondrocytes, and the efficiency of gene knockdown was examined by RT-PCR (Supp. Fig. 1). The results showed that TNF-α can stimulate calcium overload and mitochondrial dysfunction, while knockdown of NR1, the gene encoding NMDA receptor, can effectively inhibit such effects (Fig. 3A–G).
Figure 3.
Knock down of NMDA receptor ameliorates chondrocyte senescence and OA-like phenotypes. (A and D) Effect of silencing NMDA receptor (si-NR1) on the calcium level induced by TNF-α stimulated primary mouse chondrocytes (Scale bar = 10 μm). Data are presented as mean ± SEM and statistical analysis was performed using one-way ANOVA/post-hoc Bonferroni test (N = 3, *p < 0.05; **p < 0.01). (B and E-F) Representative confocal images of the mitochondrial network (Scale bar = 10 μm) and the quantified measurements of mitochondrial fragmentation (Individual counts) and mean branches per network in different groups. Data are presented as mean ± SEM and statistical analysis was performed using one-way ANOVA/post-hoc Bonferroni test (N = 5, *p < 0.05; **p < 0.01). (C and G) Mitochondrial membrane potential was detected by JC‐1 staining (Scale bar = 100 μm). The ratio of JC-1 aggregates (red) and JC‐1 monomer (green) was calculated by Image J. Data were presented as the mean ± SEM. Statistical analysis was performed using one-way ANOVA/post-hoc Bonferroni test (N = 3, *p < 0.05, **p < 0.01). (H and I) The SA-β-Gal staining (Scale bar = 100 μm) was used to determine senescent chondrocytes. The ratio of SA-β-Gal staining positive cells were semi-quantified by Image J. Data are presented as mean ± SEM, and one-way ANOVA/post-hoc Bonferroni test was used (N = 5, **p < 0.01). (J, K and L) Detection and semi-quantitative analysis of MMP13 and COL2A1 by Western blotting. Data are presented as mean ± SEM and statistical analysis was performed using one-way ANOVA/post-hoc Bonferroni test (N = 3, *p < 0.05; **p < 0.01). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Meanwhile, we found that silencing NMDA receptor resulted in a lowered percentage of senescent chondrocytes after TNF-α treatment for 72 h (Fig. 3H and I). Besides, the level of MMP13 was reduced while the level of COL2A1 was elevated after NMDA receptor knockdown in TNF-α treated chondrocytes (Fig. 3J–L). These findings suggest that NMDA receptor is involved in promoting chondrocyte senescence and OA-like phenotypes via induction of calcium overload and mitochondrial damage.
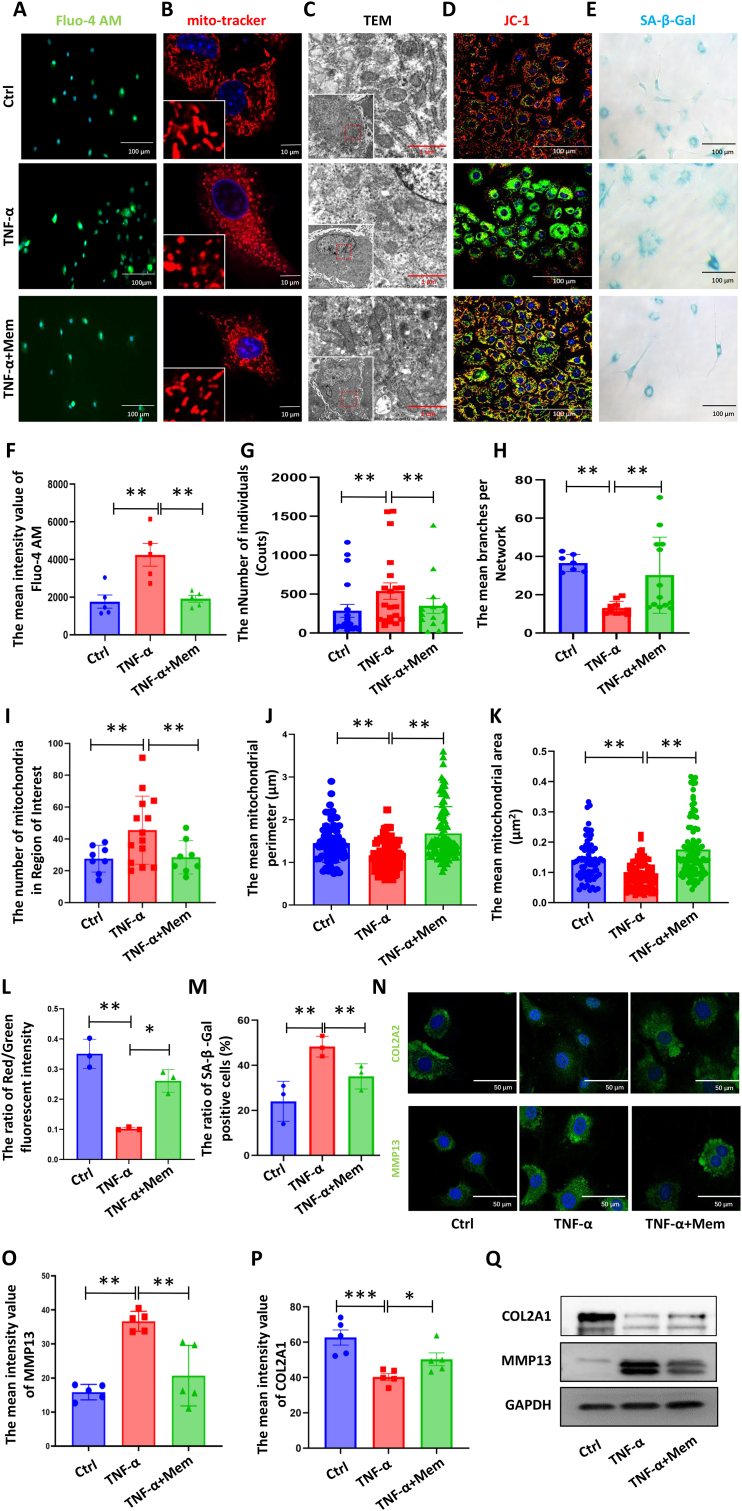
3.4. Memantine stabilizes mitochondrial function and attenuates chondrocyte senescence and OA-like phenotypes by inhibiting NMDA receptor-mediated calcium overload
To determine whether memantine is cytotoxic, the chondrocytes were treated with a series of memantine dosages (0, 5, 10, 25, 50, and 100 μM), and the cell viability was measured after 3 days. According to both previous research [22] and our results, the dosage of 10 μM memantine was selected for the subsequent experiments (Supp. Fig. 2). Then, to evaluate whether memantine can attenuate the development of OA, we constructed in vitro OA-like chondrocyte models using TNF-α with or without memantine. Inflammatory environment (TNF-α) stimulates the calcium flux in chondrocytes that can be largely reversed by memantine (Fig. 4A and F). Thereafter, we analyzed the mitochondrial damage level based on mitochondrial morphology detection. The Mito-Tracker Red CMXRos staining showed that TNF-α disturbed the mitochondrial network (Fig. 4B), manifested as increased mitochondrial staining dots (Fig. 4G) and decreased mitochondrial branches (Fig. 4H). Besides, the mitochondrial ultrastructure was also detected by TEM (Fig. 4C), and the semi-quantification results confirmed an increased number of mitochondria (Fig. 4I) but reduced mitochondrial perimeter (Fig. 4J) and area (Fig. 4K) in the TNF-α group compared with the control group. Surprisingly, memantine exhibited promising therapeutic effects in maintaining normal mitochondrial membrane potential (Fig. 4D and L).
Figure 4.
Memantine attenuates chondrocyte senescence and OA-like phenotypes induced by TNF-α. (A and F) Effect of memantine on the calcium level induced by TNF-α stimulated primary mouse chondrocytes (Scale bar = 100 μm). Data are presented as mean ± SEM and statistical analysis was performed using one-way ANOVA/post-hoc Tukey test (N = 5, **p < 0.01). (B and G-H) Representative confocal images of the mitochondrial network (Scale bar = 10 μm) and the quantified measurements of mitochondrial fragmentation (Individual counts) and mean branches per network in different groups. Data are presented as mean ± SEM and statistical analysis was performed using one-way ANOVA/post-hoc Bonferroni test (N = 5, **p < 0.01). (C and I-K) Representative TEM images of the mitochondrial morphology and the quantified measurements of mitochondrial number and size in primary mouse chondrocytes (Scale bar = 1 μm). Data are presented as mean ± SEM and statistical analysis was performed using one-way ANOVA/post-hoc Bonferroni test (N = 5, **p < 0.01). (D and L) Mitochondrial membrane potential was detected by JC‐1 staining (Scale bar = 100 μm). The ratio of JC-1 aggregates (red) and JC‐1 monomer (green) was calculated by Image J. Data were presented as the mean ± SEM. Statistical analysis was performed using one-way ANOVA/post-hoc Bonferroni test (N = 3, *p < 0.05, **p < 0.01). (E and M) The SA-β-Gal staining (Scale bar = 100 μm) was used to determine senescent chondrocytes. The ratio of SA-β-Gal staining positive cells were semi-quantified by Image J. Data are presented as mean ± SEM, and one-way ANOVA/post-hoc Bonferroni test was used (N = 3, **p < 0.01). (N–Q) Detection of MMP13 and COL2A1 treated by memantine or not in TNF-α stimulated primary mouse chondrocytes by Western blotting and immunostaining. Semi-quantification was based on immunostaining results, data are presented as mean ± SEM and statistical analysis was performed using one-way ANOVA/post-hoc Bonferroni test (N = 5, *p < 0.05, **p < 0.01, **p < 0.001). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The balance between anabolic and catabolic metabolism of chondrocytes has been recognized as the driving factor of the cartilage degeneration of OA, and chondrocyte senescence is the core cellular event in disturbing the balance [23]. In this study, IF staining and Western blotting was employed to detect the levels of COL2A1 and MMP13, the most representative cartilage anabolic and catabolic markers, respectively [23,24]. The results confirmed that memantine can inhibit the expression of the cartilage matrix degeneration marker, MMP13, but increase the expression of COL2A1 (Fig. 4N–Q). SA-β-gal staining showed that memantine can reverse the chondrocyte senescence level induced by TNF-α (Fig. 4E and M). Overall, these findings suggest that memantine has a great potential for the treatment of OA.
3.5. Memantine protects against OA in vivo
To further examine the therapeutic effects of memantine on OA progression, we established mouse OA models by performing the DMM surgery on 12-week-old male C57BL/6J mice, which were further treated with intra-articular injection of memantine (5 mg/kg) or saline twice per week for 8 weeks. The OARSI score based on the safranin O/fast green staining of mouse knee joint were used to evaluate the knee joint damage level among various groups. It was found that administration of memantine achieved significant improvement against cartilage degradation (Fig. 5A and B). As for the evaluation of chondrocyte senescence, the protein level of P16INK4a was detected [5], and the results showed that the articular cartilage from DMM mouse knee joint exhibited an increased positive staining rate of chondrocytes, which was effectively inhibited by memantine (Fig. 5C and D). Moreover, IHC staining revealed a significantly elevated level of MMP13 and a reduced level of COL2A1 in the DMM mouse knee joint, which were both greatly reversed in the memantine administration group (Fig. 5E–H). What's more, we investigated the analgesic potential of memantine in the monosodium iodoacetate (MIA) rat model of OA pain. Results showed that pain behaviors were partially eliminated by memantine intervention (Supp. Figs. 4A–E). These results confirmed our previous findings in vitro.
Figure 5.
Memantine protects against OA and memantine use is associated with a reduced risk of knee or hip OA-associated joint replacement in patients with dementia. (A) Safranin O/Fast green staining, and immunohistochemistry staining of (C) P16INK4a, (E) MMP13 and (G) COL2A1, scale bar = 100 μm. (B) Quantitative analysis of the OARSI score from (A). (D, F and H) Quantitative data for C, E and G, the IHC staining of MMP13, COL2A1 and P16INK4a. All data are normalized to the values of the DMM group, and presented as mean ± SEM. Statistical analysis was performed using one-way ANOVA/post-hoc Bonferroni test (N = 5, *p < 0.05, **p < 0.01). (I) Cumulative incidence of knee or hip OA-associated joint replacement in 1435 memantine users and 4783 AChE users, matched by 1:5 propensity score. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.6. Memantine initiation is associated with a lower risk of joint replacement in patients with OA and dementia
The flow chart illustrating the participants selection process and the baseline characteristics of each propensity score-matched cohort is shown in Supp. Fig. 3. After propensity score matching, 6218 patients (n = 1435 in memantine cohort; n = 4783 in AChE cohort) were eventually included in our analyses. The memantine cohort had a mean age of 81.5 (SD: 5.5) years and a female proportion of 59.8 %, whereas the AChE cohort had a mean age of 81.0 (SD: 5.8) years and a female proportion of 63.1 %. Overall, the characteristics were well-balanced in the propensity score-matched cohorts, with all standardized differences <0.1. The memantine initiator was found to associate with a lower risk of joint replacement than AChE initiators (Fig. 5I). As shown in Supp. Tables 1 and 14, cases of joint replacement were reported among 1435 memantine users (5.1 per 1000 person-years) and 76 cases were reported among 4783 AChE users (9.7 per 1000 person-years). The RD and HR of joint replacement for memantine versus AchE were −4.5 (95 % CI: −8.2 to −0.9) per 1000 person-years and 0.56 (95 % CI: 0.32 to 0.98), respectively. The results from sensitivity analysis (i.e., excluding subjects who had joint replacement within 3 months after the index date) did not differ substantially (Supp. Table 2)
4. Discussions
In the past few decades, enormous efforts have been devoted to elucidating the mechanisms of cartilage degeneration in OA, but no effective disease-modifying drugs have been developed yet to treat this disease [25]. As an FDA-approved drug for the treatment of dementia, memantine exerts its therapeutic effects by blocking the function of its downstream NMDA receptor in the central nervous system (CNS) [26]. However, little knowledge is known regarding the effects of memantine on articular cartilage [11]. In this study, we revealed that the expression level of NMDA receptor was increased in OA cartilage, and that the inflammatory microenvironment within articular joint can stimulate calcium overload and the subsequent OA-related phenotypic changes via the NMDA receptor. On the other hand, administration of memantine can effectively block the calcium influx mediated by NMDA receptor and reverse chondrocyte senescence and OA progression both in vitro and in vivo. Furthermore, our large population-based cohort study yielded clinical evidence that memantine use is associated with a lowered risk of knee or hip OA-related joint replacement in patients with dementia. Considering that the process of traditional drug development is time-consuming and highly costly, this study offers a strong rationale for repurposing memantine for the treatment of OA.
Notably, Zhao et al. proved that memantine can ameliorate the cartilage matrix catabolic effects induced by advance glycation end products (AGEs) on chondrocytes. The authors believed that the therapeutic effects of memantine on OA depends on its anti-inflammatory capacity, which is independent of the NMDA receptor [11,27]. However, earlier studies have already shown that glutamate and the involvement of NMDA receptor play an important role in chondrocyte apoptosis and OA development [28,29]. For instance, inhibiting the NMDA receptor via its blocker MK801, can restore the changes in circadian clock genes and reduce the expression of MMP13 in chondrocytes [30]. Therefore, whether memantine exerts its chondroprotective effects by blocking the NMDA receptor is worth clarification. As a family member of the calcium permeable ion channel, NMDA receptor activation can induce a high level of calcium influx to cause long-term potentiation of synaptic transmission in CNS [7,31]. Our study proved that the NMDA receptor in chondrocytes can be activated by inflammatory cytokines. Despite not utilizing patch clamp techniques to measure membrane electrical activity, our results still affirm the occurrence of calcium overload and the subsequent mitochondrial damage, which eventually promote chondrocyte senescence as well as OA development. These processes can be retarded by NMDA receptor knockdown or memantine use. This finding is consistent with the Lee et al., who demonstrated that intra-articular MgSO4 injection inhibited NMDA receptor and suppressed OA cartilage degeneration [28]. Besides, since peripheral nerve tissue is also involved in OA, we cannot rule out the possibility that memantine may alleviate OA through regulating peripheral nervous system within the joint.
Interestingly, NMDA receptor also plays a crucial role in the development and chronification of pain, and its antagonists have recently surfaced as a pharmacological pain management strategy, especially in dealing with neuropathic pain [32]. Pain is the dominant symptom and the major driver of clinical decision making and health service use for OA patients. Apart from peripheral nociceptive pain mechanisms, neuropathic pain or central pain mechanisms also seem to take effect in a large proportion of patients with OA [33]. Therefore, the analgesia function of NMDA receptor antagonists in OA has drawn wide attention. Lee et al. applied MgSO4 to suppress NMDA receptor, which resulted in a significant improvement of synovitis, mechanical allodynia and thermal hyperalgesia in the OA + MgSO4 group compared with the OA group [28]. Plant species including Chenopodium ambrosioides L. (Amaranthaceae) were used as NMDA antagonists to treat OA pain in another study, which yielded results that were consistent with those obtained by Lee et al. [34]. Moreover, NMDA receptor activation in peripheral and central nervous systems is the main cause of sensitization and chronification of OA pain [35,36]. Thus, there is extensive evidence to support that drug repurposing of memantine can not only prevent cartilage degeneration but also help with OA pain management.
Our study provides a comprehensive understanding of the efficacy and underlying mechanism of memantine in OA treatment, but the safety of memantine in the treatment of OA still needs further exploration. Specifically, several serious adverse events such as congestive heart failure, acute renal failure, Stevens Johnson syndrome, etc. should be closely monitored [37]. Besides, since OA has been traditionally characterized as a wear and tear disease, the role of NMDA receptor in mechanical loading is also a topic of interest. Calcium-permeable ion channel proteins such as transient potential receptor vanillin 4 channel (TRPV4) and Piezo are important mechanosensors in chondrocytes that can convert mechanical signals into biochemical signals [38]. Salter et al. proved that the NMDA receptor antagonists inhibited the hyperpolarization response of normal chondrocytes to mechanical stimulation [10], suggesting that NMDA receptor may be also involved in the regulation of human articular chondrocyte responses to mechanical stimulation [39]. Nonetheless, the exact role of NMDA receptor in cartilage degradation induced by mechanical overload and whether memantine can also protect chondrocytes under excess mechanical stimuli still need to be further elucidated.
5. Conclusion
NMDA receptor plays an important role in inflammatory-induced cytotoxic calcium overload in chondrocytes, while memantine can effectively block the NMDA receptor to reduce chondrocyte senescence and retard the development of OA. These findings present robust evidence for repurposing memantine for the treatment of OA.
Author contributions
Drs. Wang, Li, and Wei had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Wang, Li, and Wei are joint corresponding authors. All authors have read, provided critical feedback on intellectual content and approved the final manuscript. Concept and design: Wang, Li, and Wei. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: Wang, Cheng, Li and Wei. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Cheng, and Wei. Obtained funding: Wang, Li, and Wei. Administrative, technical, or material support: Wang, Li and Wei. Supervision: Wang, Li and Wei.
Ethical approval
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of the National Institutes of Health and approved by the medical ethical committee of Xiangya Hospital of Central South University (2022020438). All relevant guidelines for the work with animals were adhered in this study. The human cohort study received approval from the medical ethical committee of Xiangya Hospital (2018091077).
Patient consent
Human primary cells were obtained from ten patients with OA ranging in age from 63 to 78 years (3 males, 7 females) during total knee replacement surgery. Patients with rheumatoid arthritis, metabolic disease, or other inflammatory diseases were excluded. All participants provided informed consent. We obtained a waiver of informed consent from the medical ethical committee of Xiangya Hospital (202110186) for the human cohort study.
Funding/support statement
This work was supported by the National Natural Science Foundation of China (82202769), the Natural Science Foundation of Hunan Province (2023JJ40993), the science and technology innovation Program of Hunan Province (2023RC3076), the Natural Science Foundation of Hunan Province (2022JJ20100), Young Scientific Talent of Hunan Province (2022RC1009), and Scientific Research Program of FuRong Laboratory (2023SK2100). No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Role of the funder/sponsor
The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Patient and public involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for design or implementation of the study. No patients were asked to advise on interpretation or writing up of results. Dissemination of the findings to participants is not possible owing to the use of an anonymised dataset.
Scientific approval
This study was approved by the IMRD Scientific Review Committee (21SRC054-A1).
Statement
THIN is a registered trademark of Cegedim SA in the United Kingdom and other countries. Reference made to the THIN database is intended to be descriptive of the data asset licensed by IQVIA. This work uses de-identified data provided by patients as a part of their routine primary care.
Disclaimer
The interpretation of these data is the sole responsibility of the authors.
Transparency
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Conflict of interest
No conflict of interest for any of the authors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Everyone who contributed significantly to the work has been listed.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2024.08.007.
Contributor Information
Ning Wang, Email: znxywn@csu.edu.cn.
Hui Li, Email: lihui1988@csu.edu.cn.
Jie Wei, Email: weij1988@csu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
The NMDA receptor knockdown in chondrocytes determined by RT-PCR.
The efficiency of NMDA receptor knockdown in chondrocytes was determined by RT-PCR. Data are presented as mean ± SEM and statistical analysis was performed using Student's t test comparing with Ctrl group (N = 3, **p < 0.01).
Memantine regulates chondrocyte viability.
Cell viability was measured for the mouse chondrocytes treated by memantine (0, 5, 10, 25 and 50 μM) with (B) or without (A) TNF-α for 1–3 days. Data are presented as mean ± SEM and analyzed by two-way ANOVA and multiple t test, * compares to Ctrl group, # compares to TNF-α group (N = 5, *p < 0.05, **p < 0.01, #p < 0.05).
The flow chart of participants selection and validation.
Memantine treatment effectively alleviates pain in vivo.
Pain sensitivity and mobility measured by von Frey filaments (A), hotplate analysis (B) and weight bearing (C) monitor two times a week after MIA intraarticular injection. Immunofluorescent staining of CGRP (D), scale bar=100 µm, Data are presented as mean ± SEM, and one-way ANOVA/post-hoc bonferroni test was used (N=5, *p < 0.05). For pain behavior measurement, statistical analysis was conducted using two-way ANOVA/post-hoc Tukey test. Data are mean ± SEM, P values were compared between Sham and MIA group (N=6, *p < 0.05, **p < 0.01, ***p < 0.001), MIA and MIA + Memantine group (N=6, #p < 0.05, ##p < 0.01).
Baseline characteristics of memantine initiation and AChE initiation in patients with OA and Dementia (≥40 years).
Data are presented as mean ± SD, median (IQR), or n (%). Abbreviations: BMI: body mass index; BMI, body mass index; n, number; y, years; SD, standard deviation; OA, osteoarthritis; ACE, angiotensin converting enzyme; PPIs, proton pump inhibitors.
†The Socio-Economic Deprivation Index (i.e., Townsend Deprivation Index) was grouped into quintiles from 1 (least deprived) to 5 (most deprived).
‡Frequency during the past one year.
Association between memantine initiation and risk of joint replacement comparing with initiation of AChE in patients with OA and Dementia.
References
- 1.Loeser R.F., Collins J.A., Diekman B.O. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12(7):412–420. doi: 10.1038/nrrheum.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie J., Wang Y., Lu L., Liu L., Yu X., Pei F. Cellular senescence in knee osteoarthritis: molecular mechanisms and therapeutic implications. Ageing Res Rev. 2021;70 doi: 10.1016/j.arr.2021.101413. [DOI] [PubMed] [Google Scholar]
- 3.Wang N., Zhang X., Rothrauff B.B., Fritch M.R., Chang A., He Y., et al. Novel role of estrogen receptor-α on regulating chondrocyte phenotype and response to mechanical loading. Osteoarthritis Cartilage. 2022;30(2):302–314. doi: 10.1016/j.joca.2021.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Coryell P.R., Diekman B.O., Loeser R.F. Mechanisms and therapeutic implications of cellular senescence in osteoarthritis. Nat Rev Rheumatol. 2021;17(1):47–57. doi: 10.1038/s41584-020-00533-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeon O.H., Kim C., Laberge R.M., Demaria M., Rathod S., Vasserot A.P., et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23(6):775–781. doi: 10.1038/nm.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koola M.M. Galantamine-Memantine combination in the treatment of Alzheimer's disease and beyond. Psychiatr Res. 2020;293 doi: 10.1016/j.psychres.2020.113409. [DOI] [PubMed] [Google Scholar]
- 7.Song X., Jensen M., Jogini V., Stein R.A., Lee C.H., McHaourab H.S., et al. Mechanism of NMDA receptor channel block by MK-801 and memantine. Nature. 2018;556(7702):515–519. doi: 10.1038/s41586-018-0039-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garbincius J.F., Elrod J.W. Mitochondrial calcium exchange in physiology and disease. Physiol Rev. 2022;102(2):893–992. doi: 10.1152/physrev.00041.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang S., Li W., Zhang P., Wang Z., Ma X., Liu C., et al. Mechanical overloading induces GPX4-regulated chondrocyte ferroptosis in osteoarthritis via Piezo1 channel facilitated calcium influx. J Adv Res. 2022;41:63–75. doi: 10.1016/j.jare.2022.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salter D.M., Wright M.O., Millward-Sadler S.J. NMDA receptor expression and roles in human articular chondrocyte mechanotransduction. Biorheology. 2004;41(3–4):273–281. [PubMed] [Google Scholar]
- 11.Zhao J., Yu Y., Wu Z., Wang L., Li W. Memantine inhibits degradation of the articular cartilage extracellular matrix induced by advanced glycation end products (AGEs) Biomed Pharmacother. 2017;91:1193–1198. doi: 10.1016/j.biopha.2017.04.054. [DOI] [PubMed] [Google Scholar]
- 12.Li H., Ding X., Terkeltaub R., Lin H., Zhang Y., Zhou B., et al. Exploration of metformin as novel therapy for osteoarthritis: preventing cartilage degeneration and reducing pain behavior. Arthritis Res Ther. 2020;22(1):34. doi: 10.1186/s13075-020-2129-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lurette O., Guedouari H., Morris J.L., Martín-Jiménez R., Robichaud J.P., Hamel-Côté G., et al. Mitochondrial matrix-localized Src kinase regulates mitochondrial morphology. Cell Mol Life Sci. 2022;79(6):327. doi: 10.1007/s00018-022-04325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Chen F, Wu X, Bian H, Chen C, Zhang X, et al. DLX5 promotes Col10a1 expression and chondrocyte hypertrophy and is involved in osteoarthritis progression. Genes Dis. 2023;10(5):2097–2108. doi: 10.1016/j.gendis.2022.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Wet S., Mangali A., Batt R., Kriel J., Vahrmeijer N., Niehaus D., et al. The highs and lows of memantine-an autophagy and mitophagy inducing agent that protects mitochondria. Cells. 2023;12(13) doi: 10.3390/cells12131726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez-Coria H., Green K.N., Billings L.M., Kitazawa M., Albrecht M., Rammes G., et al. Memantine improves cognition and reduces Alzheimer's-like neuropathology in transgenic mice. Am J Pathol. 2010;176(2):870–880. doi: 10.2353/ajpath.2010.090452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Courties A., Olmer M., Myers K., Ordoukhanian P., Head S.R., Natarajan P., et al. Human-specific duplicate CHRFAM7A gene is associated with more severe osteoarthritis and amplifies pain behaviours. Ann Rheum Dis. 2023;82(5):710–718. doi: 10.1136/ard-2022-223470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun J., Song F.H., Wu J.Y., Zhang L.Q., Li D.Y., Gao S.J., et al. Sestrin2 overexpression attenuates osteoarthritis pain via induction of AMPK/PGC-1α-mediated mitochondrial biogenesis and suppression of neuroinflammation. Brain Behav Immun. 2022;102:53–70. doi: 10.1016/j.bbi.2022.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Li H., Zhou B., Wu J., Zhang Y., Zhang W., Doherty M., et al. Melatonin is a potential novel analgesic agent for osteoarthritis: evidence from cohort studies in humans and preclinical research in rats. J Pineal Res. 2024;76(2) doi: 10.1111/jpi.12945. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X., Xiang S., Zhang Y., Liu S., Lei G., Hines S., et al. In vitro study to identify ligand-independent function of estrogen receptor-α in suppressing DNA damage-induced chondrocyte senescence. Faseb j. 2023;37(2) doi: 10.1096/fj.202201228R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang K.D., Ding X., Jiang N., Zeng C., Wu J., Cai X.Y., et al. Digoxin targets low density lipoprotein receptor-related protein 4 and protects against osteoarthritis. Ann Rheum Dis. 2022;81(4):544–555. doi: 10.1136/annrheumdis-2021-221380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santos Souza H.F., Rocha S.C., Damasceno F.S., Rapado L.N., Pral E.M.F., Marinho C.R.F., et al. The effect of memantine, an antagonist of the NMDA glutamate receptor, in in vitro and in vivo infections by Trypanosoma cruzi. PLoS Negl Trop Dis. 2019;13(9) doi: 10.1371/journal.pntd.0007226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otsuki S., Hanson S.R., Miyaki S., Grogan S.P., Kinoshita M., Asahara H., et al. Extracellular sulfatases support cartilage homeostasis by regulating BMP and FGF signaling pathways. Proc Natl Acad Sci U S A. 2010;107(22):10202–10207. doi: 10.1073/pnas.0913897107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagata K., Hojo H., Chang S.H., Okada H., Yano F., Chijimatsu R., et al. Runx2 and Runx3 differentially regulate articular chondrocytes during surgically induced osteoarthritis development. Nat Commun. 2022;13(1):6187. doi: 10.1038/s41467-022-33744-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao Q., Wu X., Tao C., Gong W., Chen M., Qu M., et al. Osteoarthritis: pathogenic signaling pathways and therapeutic targets. Signal Transduct Target Ther. 2023;8(1):56. doi: 10.1038/s41392-023-01330-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reisberg B., Doody R., Stöffler A., Schmitt F., Ferris S., Möbius H.J. Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med. 2003;348(14):1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 27.Chen S.L., Tao P.L., Chu C.H., Chen S.H., Wu H.E., Tseng L.F., et al. Low-dose memantine attenuated morphine addictive behavior through its anti-inflammation and neurotrophic effects in rats. J Neuroimmune Pharmacol. 2012;7(2):444–453. doi: 10.1007/s11481-011-9337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee C.H., Wen Z.H., Chang Y.C., Huang S.Y., Tang C.C., Chen W.F., et al. Intra-articular magnesium sulfate (MgSO4) reduces experimental osteoarthritis and nociception: association with attenuation of N-methyl-D-aspartate (NMDA) receptor subunit 1 phosphorylation and apoptosis in rat chondrocytes. Osteoarthritis Cartilage. 2009;17(11):1485–1493. doi: 10.1016/j.joca.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Piepoli T., Mennuni L., Zerbi S., Lanza M., Rovati L.C., Caselli G. Glutamate signaling in chondrocytes and the potential involvement of NMDA receptors in cell proliferation and inflammatory gene expression. Osteoarthritis Cartilage. 2009;17(8):1076–1083. doi: 10.1016/j.joca.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Kalev-Zylinska M.L., Hearn J.I., Rong J., Zhu M., Munro J., Cornish J., et al. Altered N-methyl D-aspartate receptor subunit expression causes changes to the circadian clock and cell phenotype in osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2018;26(11):1518–1530. doi: 10.1016/j.joca.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Alkadhi K.A. NMDA receptor-independent LTP in mammalian nervous system. Prog Neurobiol. 2021;200 doi: 10.1016/j.pneurobio.2020.101986. [DOI] [PubMed] [Google Scholar]
- 32.Niesters M., Dahan A. Pharmacokinetic and pharmacodynamic considerations for NMDA receptor antagonists in the treatment of chronic neuropathic pain. Expert Opin Drug Metab Toxicol. 2012;8(11):1409–1417. doi: 10.1517/17425255.2012.712686. [DOI] [PubMed] [Google Scholar]
- 33.Hunter D.J., Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–1759. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 34.Calado G.P., Lopes A.J., Costa Junior L.M., Lima F., Silva L.A., Pereira W.S., et al. Reduces synovial inflammation and pain in experimental osteoarthritis. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0141886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X., Zhou W., Wang L., Ye Y., Li T. Transcranial direct current stimulation alleviates the chronic pain of osteoarthritis by modulating NMDA receptors in midbrain periaqueductal gray in rats. J Pain Res. 2022;15:203–214. doi: 10.2147/JPR.S333454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meng Y., Shen H.L. Role of N-Methyl-D-Aspartate receptor NR2B subunit in inflammatory arthritis-induced chronic pain and peripheral sensitized neuropathic pain: a systematic review. J Pain Res. 2022;15:2005–2013. doi: 10.2147/JPR.S367982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elnaiem W., Benmelouka A.Y., Elgendy A.M.N., Abdelgalil M.S., Brimo Alsaman M.Z., Mogheeth A., et al. Evaluation of memantine's efficacy and safety in the treatment of children with autism spectrum disorder: a systematic review and meta-analysis. Hum Psychopharmacol. 2022;37(5) doi: 10.1002/hup.2841. [DOI] [PubMed] [Google Scholar]
- 38.Wang N., Lu Y., Rothrauff B.B., Zheng A., Lamb A., Yan Y., et al. Mechanotransduction pathways in articular chondrocytes and the emerging role of estrogen receptor-α. Bone Res. 2023;11(1):13. doi: 10.1038/s41413-023-00248-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang K., Wang L., Liu Z., Geng B., Teng Y., Liu X., et al. Mechanosensory and mechanotransductive processes mediated by ion channels in articular chondrocytes: potential therapeutic targets for osteoarthritis. Channels. 2021;15(1):339–359. doi: 10.1080/19336950.2021.1903184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The NMDA receptor knockdown in chondrocytes determined by RT-PCR.
The efficiency of NMDA receptor knockdown in chondrocytes was determined by RT-PCR. Data are presented as mean ± SEM and statistical analysis was performed using Student's t test comparing with Ctrl group (N = 3, **p < 0.01).
Memantine regulates chondrocyte viability.
Cell viability was measured for the mouse chondrocytes treated by memantine (0, 5, 10, 25 and 50 μM) with (B) or without (A) TNF-α for 1–3 days. Data are presented as mean ± SEM and analyzed by two-way ANOVA and multiple t test, * compares to Ctrl group, # compares to TNF-α group (N = 5, *p < 0.05, **p < 0.01, #p < 0.05).
The flow chart of participants selection and validation.
Memantine treatment effectively alleviates pain in vivo.
Pain sensitivity and mobility measured by von Frey filaments (A), hotplate analysis (B) and weight bearing (C) monitor two times a week after MIA intraarticular injection. Immunofluorescent staining of CGRP (D), scale bar=100 µm, Data are presented as mean ± SEM, and one-way ANOVA/post-hoc bonferroni test was used (N=5, *p < 0.05). For pain behavior measurement, statistical analysis was conducted using two-way ANOVA/post-hoc Tukey test. Data are mean ± SEM, P values were compared between Sham and MIA group (N=6, *p < 0.05, **p < 0.01, ***p < 0.001), MIA and MIA + Memantine group (N=6, #p < 0.05, ##p < 0.01).
Baseline characteristics of memantine initiation and AChE initiation in patients with OA and Dementia (≥40 years).
Data are presented as mean ± SD, median (IQR), or n (%). Abbreviations: BMI: body mass index; BMI, body mass index; n, number; y, years; SD, standard deviation; OA, osteoarthritis; ACE, angiotensin converting enzyme; PPIs, proton pump inhibitors.
†The Socio-Economic Deprivation Index (i.e., Townsend Deprivation Index) was grouped into quintiles from 1 (least deprived) to 5 (most deprived).
‡Frequency during the past one year.
Association between memantine initiation and risk of joint replacement comparing with initiation of AChE in patients with OA and Dementia.