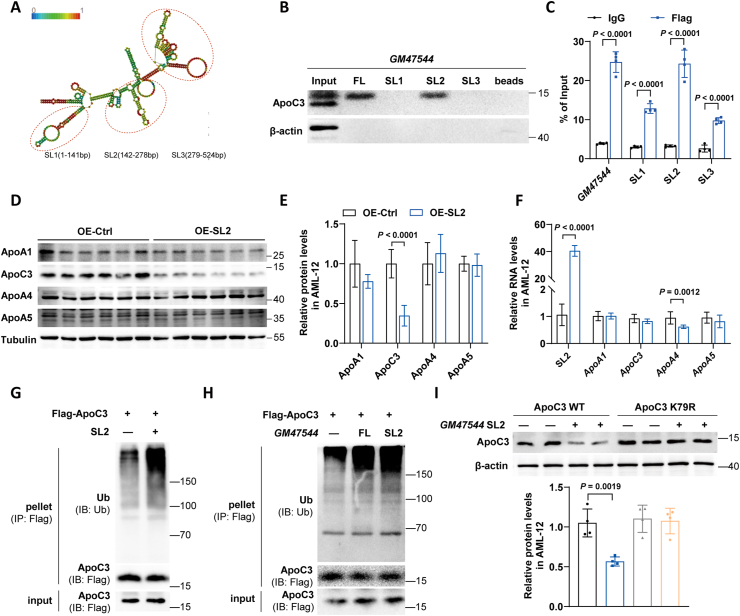
Figure 6.
GM47544 SL2 directly interacted with ApoC3 and promoted the degradation of ApoC3.
(A) The predicted secondary structure of GM47544 RNA.
(B) Invitro-synthesized full-length (FL), stem-loop structure1 (SL1), stem-loop structure2 (SL2) and stem-loop structure3 (SL3) fragments of GM47544 were incubated with AML-12 cell protein lysates. Subsequently, RNA pull-down and Western blotting assays were performed.
(C) RIP assays were performed using an anti-Flag antibody in AML-12 cells stably transfected with Flag-ApoC3. RT-qPCR analysis was employed to measure the enrichment of GM47544 and its fragments.
(D–E) ApoC3 protein levels were measured by western blot in AML-12 cells transfected with pc3.1 (OE-Ctrl) or pc3.1-GM47544 SL2 (OE-SL2).
(F) Relative expression of GM47544 and ApoA1/C3/A4/A5 were measured by RT-qPCR in control and GM47544 SL2-overexpressed AML-12 cells.
(G) IP assays were performed on AML-12 cells transfected with vectors expressing FLAG-ApoC3 and pc3.1-GM47544 SL2 using anti-FLAG antibodies, followed by western blotting analysis with antibodies against ubiquitin.
(H) IP assays were performed on AML-12 cells transfected with vectors expressing FLAG-ApoC3, pc3.1-GM47544, or pc3.1-GM47544 SL2 using anti-FLAG antibodies, followed by western blotting analysis with antibodies against ubiquitin.
(I) With or without GM47544 full length or SL2, ApoC3 proteins were measured by western blot in AML-12 cells transfected with ApoC3 WT or ApoC3 K79R.
Data are presented as mean ± SD in C, E, F, and I, with statistical significance determined with unpaired two-tailed Student's t-test.