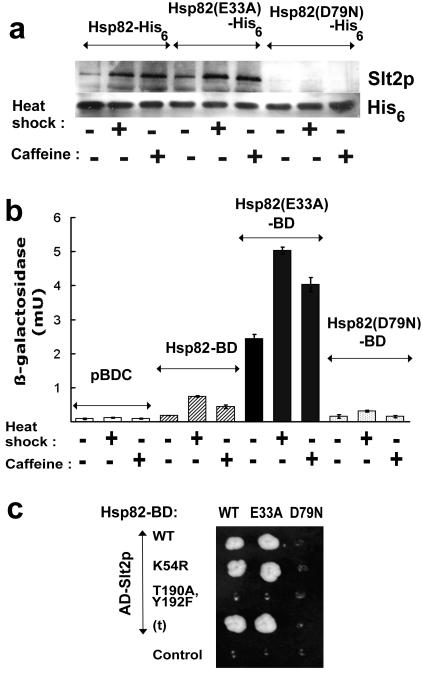
FIG. 3.
Effects of heat shock and caffeine stress on interaction of Hsp82 with the Slt2p MAP kinase. (a) Analysis of the level of Hsp82-His6-bound Slt2p in extracts from unstressed (25°C), heat shocked (from 25 to 39°C for 1 h), and caffeine-treated (8 mM for 1 h) cells expressing either a wild-type, an E33A mutant, or a D79N mutant Hsp82-His6. The Western blot was probed with anti-Slt2p and anti-His6 antisera. (b) Quantitation of the strength of Hsp82-BD-AD-Slt2p interaction (measurements of interaction-responsive lacZ expression in strain PJ694), showing that the Hsp82-BD-AD-Slt2p interaction in the two-hybrid system is moderately reinforced by stress, strongly reinforced by the E33A mutation, which inhibits the ATPase reaction of Hsp82, but abolished by the D79N mutation, inhibiting ATP/ADP binding by the chaperone. The control cells (pBDC) expressed AD-Slt2p but contained empty pBDC, since basal lacZ expression in this system is generally due to the AD fusion (37). (c) 3-AT-resistant growth of the same PJ694 cells expressing the wild-type (WT) or the E33A or D79N mutant form of Hsp82-BD in conjunction with either a wild-type AD-Slt2p fusion (WT), a nonphosphorylatable (T190A,Y192F) or phosphorylatable, yet kinase-dead (K54R), mutant form of this AD-Slt2p, or a C terminally truncated AD-Slt2p [AD-Slt2(t)p] (t). Growth was for 16 days at 30°C on DO minus leucine, tryptophan, and histidine and supplemented with 2 mM 3-AT.