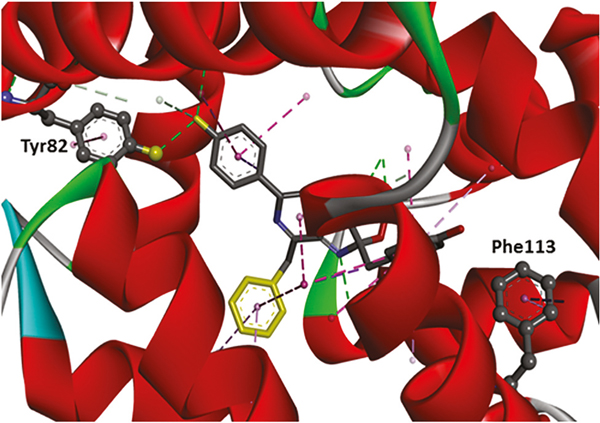
Fig. 2.
Three-dimensional crystal structure of aequorin with the locations of the Tyr82 and Phe113 highlighted. The dotted lines display the interactions between aequorin and coelenterazine. The tyrosine residue at position 82 was substituted by phenylalanine to decrease the H-bond interaction, and thus red-shifted the emission wavelength. On the other hand, the phenylalanine at position 113 was substituted by tryptophan to increase the π-π interaction, and therefore blue-shifted the emission wavelength.