FIGURE 4.
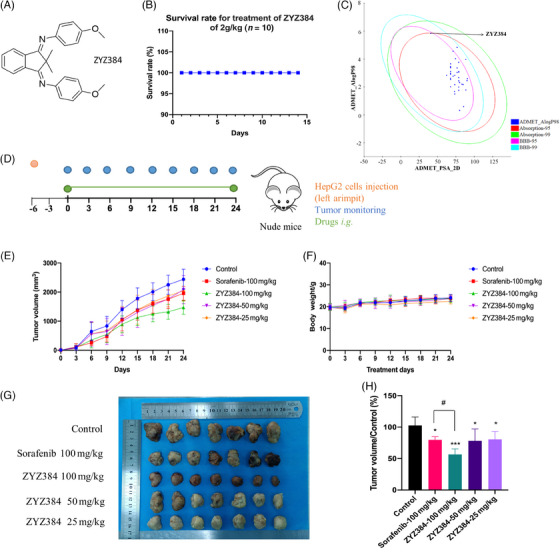
In vivo study and druggability prediction of ZYZ384. (A) The structure of ZYZ384. (B) Safety and attempted evaluation of lethal dose for ZYZ384. C57BL/6 mice were orally administrated with 2 g/kg (n = 10) of ZYZ384 for 14 days, and the survival of the mice was monitored and recorded. (C) ADMET analysis of novel small molecules. Discovery Studio software (2020) was used to calculate ADMET properties. A, Absorption; D, Distribution; M, Metabolism; E, Excretion; T, Toxicity, the 99% and 95% confidence intervals of the blood–brain barrier permeability (BBB) model, and the 99% and 95% confidence intervals of the human intestinal absorption (HIA) model. (D) The establishment of tumor‐bearing nude mice model and treatment method. (E) Tumor volume monitoring during treatment. (F) Body weight monitoring during treatment. (G) Tumor volume of each group. (H) Comparison of tumor volume in each group. Data were expressed as mean ± SEM. ADMET, Absorption, Distribution, Metabolism, Excretion, Toxicity. #: p < 0.05 versus positive control; ***: p < 0.001; *: p < 0.05 versus control, one‐way ANOVA analysis.
