Figure 6. CG9297/thinman is regulated by CRTC in the heart.
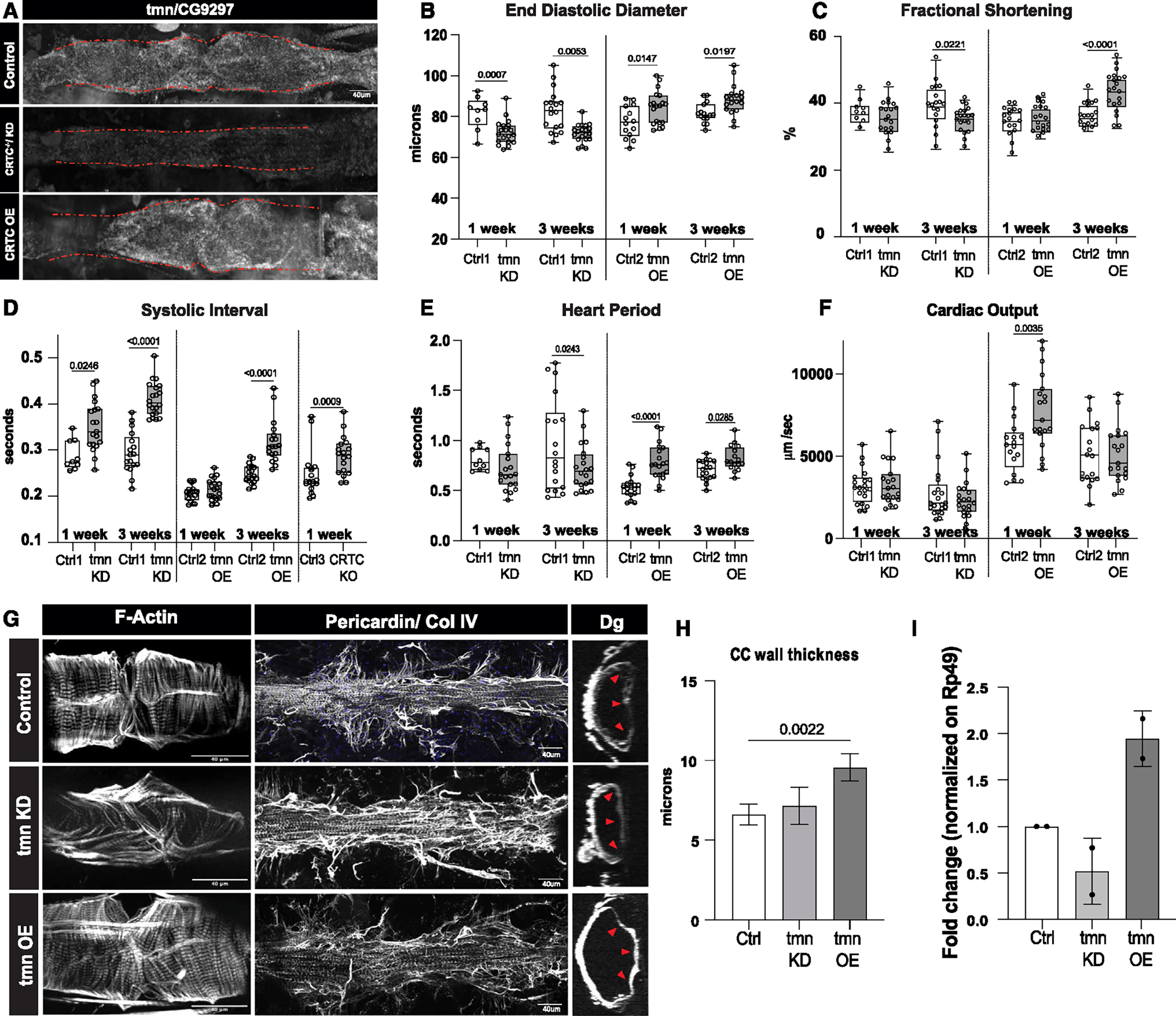
(A) HCR in Drosophila hearts shows that tmn (CG9297)/Srl is ubiquitously expressed in control hearts (tinCΔ4-Ga4/+); expression was significantly reduced in the CRTC mutant and KD hearts, while expression was increased in CRTC-OE hearts. Scale bar, 40 μm
(B) (Left) EDD was significantly reduced in response to cardiac tmn/Srl KD at both 1 and 3 week of age compared to controls. (Right) EDD was significantly increased in response to cardiac tmn/Srl OE at both 1 and 3 week of age compared to controls. (For B–F, Crtl1 flies were tinCΔ4-Ga4/+ [GD Cntrl], Ctrl2 flies were tinCΔ4-Ga4/w1118, and Ctrl3 flies were w1118).
(C) (Left) Fractional shortening was reduced by cardiac tmn/Srl KD at 3 week of age compared to controls (+). (Right) Fractional shortening increased in response to cardiac tmn/SRL OE at 3 week of age compared to controls.
(D) (Left) Systolic intervals are increased in response to cardiac tmn/Srl KD at both 1 and 3 week of age compared to wild-type controls (+). (Middle) Systolic intervals are increased in response to cardiac tmn/SRL OE at 3 week of age compared to controls. (Right) Systolic intervals in fly hearts are increased in response to cardiac-specific KO of CRTC at 1 week of age compared to controls.
(E) (Left) Heart period was decreased in response to cardiac tmn/Srl KD at 3 week of age compared to controls (+). (Right) Heart period was also significantly increased in response to cardiac tmn/Srl OE at both 1 and 3 week of age compared to controls.
(F) (Left) Cardiac output was not significantly affected by cardiac tmn/Srl KD. (Right) Cardiac output was significantly increased in response to cardiac tmn/Srl OE at 1 week of age. (Plots show all data points, max, min, median, and p values; significance by one-way ANOVA with Tukey’s multiple comparisons post hoc test.)
(G) Compared to the closely packed myofibrils in controls (top), F-actin staining shows disorganized myofibrils in tmn/Srl-KD hearts (middle), but relatively normal circumferential myofibrillar arrangement in tmn/Srl-OE hearts (bottom). Anti-pericardin (collagen IV) staining shows a more extensive collagen network surrounding the tmn/Srl-KD heart compared to control. Dystroglycan staining followed by optical sectioning showed an increased thickness in tmn/Srl-OE hearts, especially noticeable at arrowheads. (Dorsal is right, ventral is left.) (For G–I, control flies were tinCΔ4-Ga4/+). Scale bars, 40 μm.
(H) Myocardial cell thickness, made at three points along the heart tube (red arrowheads in G), was significantly increased by cardiac-specific tmn/Srl-OE. (Significance by one-way ANOVA and Tukey’s multiple comparisons post hoc test; p value shown).
(I) RT-qPCR quantification of tmn expression in isolated hearts from genetic background controls (+) and cardiac tmn-KD and -OE hearts. (Normalized to ribosomal protein 49, two biological replicates in triplicate).
