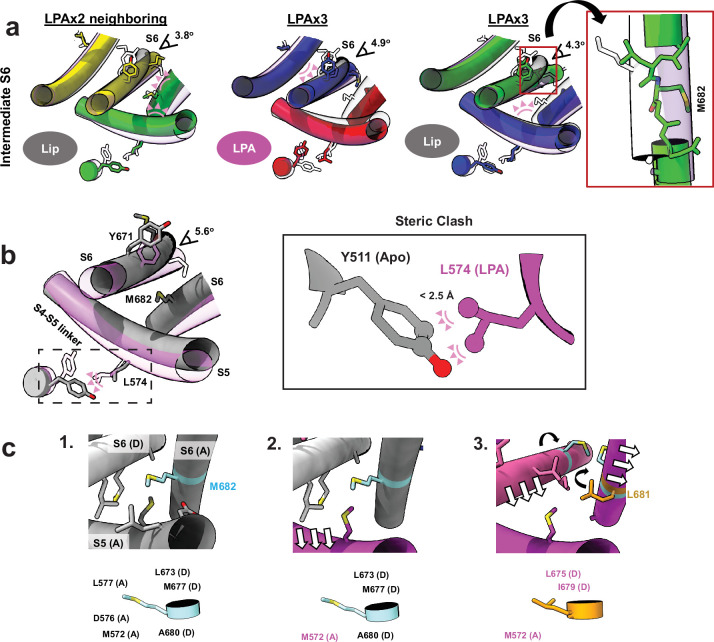
Extended Data Fig. 8. Intermediate states and mechanism of allostery.
(a) Intermediate states of the S6 helices. Steric clashes preventing full opening of the S6 helix are shown as pink triangles. Angles of opening are shown as a measure of the intermediate state. For LPAx3, the S6-C helix is distorted from a typical α-helical structure. (b) Steric clash between the superimposed position of Y511 in the closed state (grey) and L574 in the LPAx4 state (magenta) shows that the inward movement of Y511 is necessary for open transitions to occur. Boxed figure shows a zoom-in of the steric clash represented as pink pseudobonds (<2.5 Å distance). (c) Proposed mechanism for the LPA-induced opening of TRPV1 as the channel goes from apo state (grey) to one completely occupied by LPA (magenta). Residues in contact with M682 (< 5.0 Å) as it switches positions with L681 due to the π-α helix transition are highlighted. 1. M682 maintains contact with several hydrophobic residues in the apo state. 2. The S4-S5 linker moves inward toward the pocket because of Y511 flipping toward the pocket with LPA bound; the space around M682 opens. 3. S5 and S6 dilate away from the pore axis because of the void created by the moving of the S4-S5 linker; M682 is able to rotate towards the pore axis and the π-α transition occurs.