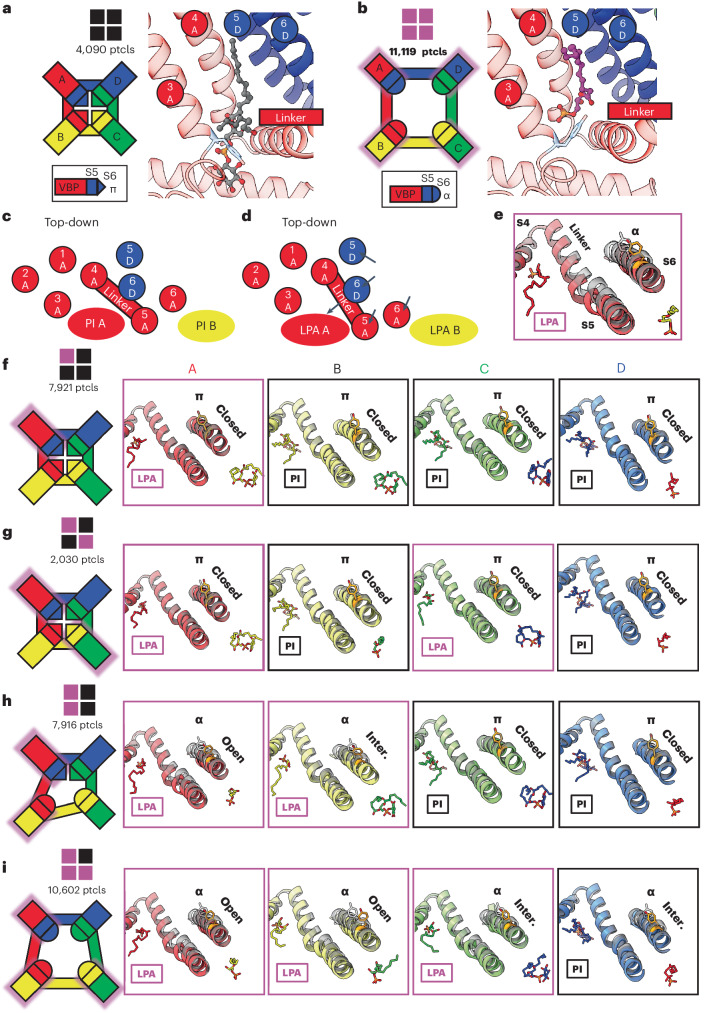
Fig. 5. Substoichiometric states of LPA binding.
a, The closed configuration of TRPV1 with all four subunits occupied by PI lipid. Particle numbers (ptcls) are shown. b, The open configuration of TRPV1 with LPA bound to all four subunits. The VBPs of the A monomers are shown to demonstrate the domain-swap architecture that comprises the VBP (S3, S4 and S4–S5 from A; S5 and S6 from D). c,d, Schematic of the binding pocket from a top-down view highlighting the domain-swap architecture and helical movements between the closed (c) and open (d) conformations. e, Structural changes associated with the binding of LPA compared to apo (transparent black). f–i, Substochiometric states of LPA binding with monomers bound with 1 LPA (f), 2 LPA in opposite pockets (g), 2 LPA in neighboring pockets (h) and 3 LPA (i). The leftmost panels show a cartoon representation of the TRPV1 tetramer indicating the functional state of each VBP monomer. Monomers are labeled anticlockwise; LPA-occupied monomers are shadowed with magenta. The functional state of the pore is indicated as ‘closed’ (π helix at Y671), ‘open’ (α helix at Y671 with S6 moved away from the pore to the same extent as fully occupied LPA) and ‘intermediate’ (‘inter.’, α helix at Y671 with S6 positioned between that of apo and fully occupied LPA).