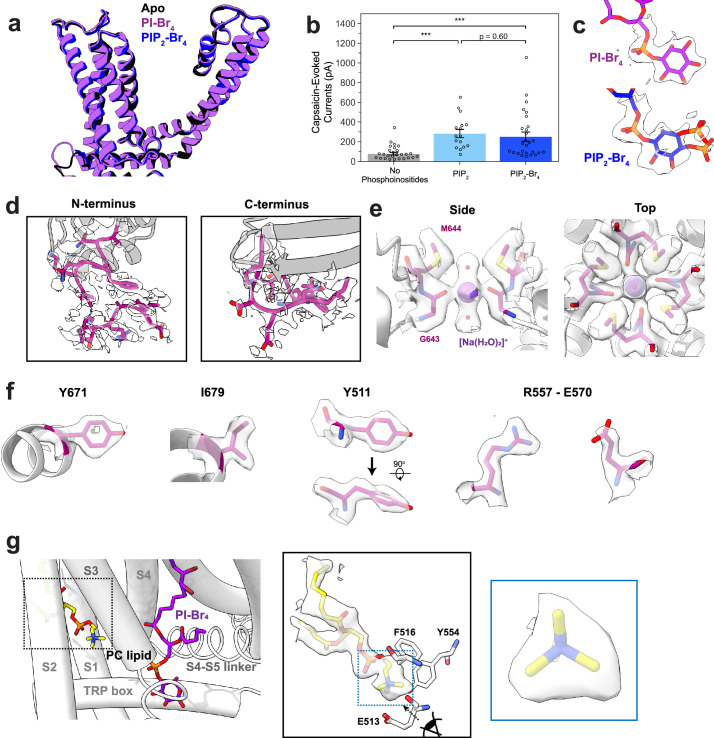
Extended Data Fig. 2. Structural details revealed by the brominated lipid data.
(a) Superimposition of the transmembrane region of TRPV1 in the apo (black), PI-Br4-bound (purple), and PIP2-Br4-bound (blue) states. (b) Patch clamp summary of TRPV1 purified and reconstituted into defined liposomes, in conditions without phosphoinositides (patch clamp recordings, n = 27), with PIP2 (n = 16), or PIP2-Br4 (n = 27) added. Currents were evoked with 10 µM capsaicin at a membrane potential of +120 mV. Hollow circles represent individual data points, bar graph indicates mean ± S.E.M. for the condition. Statistical significance was determined by Kruskal-Wallis test (H = 24.8, p = 4.2 × 10−6) and post-hoc Dunn’s test using Bonferroni correction (p = 1.6 × 10−5 for No Phosphoinositides vs PIP2, p = 4.6 × 10−4 for No Phosphoinositides vs PIP2-Br4, p = 0.60 for PIP2 vs PIP2-Br4), *** p < 0.001. (c) Lipid headgroup densities for PI-Br4 and PIP2-Br4. One monomer is shown for clarity. (d) Higher resolution for the N-terminus and C-terminus of the PI-Br4 consensus map allows for further modeling of the N-terminus (starting at residue 177) and the remaining C-terminus of the truncated TRPV1 construct (up to residue 764). (e) Captured density at the selectivity filter in the PI-Br4 consensus map models well to a [Na(H2O)2]+ cation coordinated to G643. (f) Well-defined densities for key residues as revealed in the PIBr4 consensus map. (g) Lipid density in a binding pocket within the S1 to S3 transmembrane helices shows well-defined features for a PC headgroup, importantly the tri-lobular of the quaternary amine group. Data shown is from the PI-Br4 data in Conformation 1.