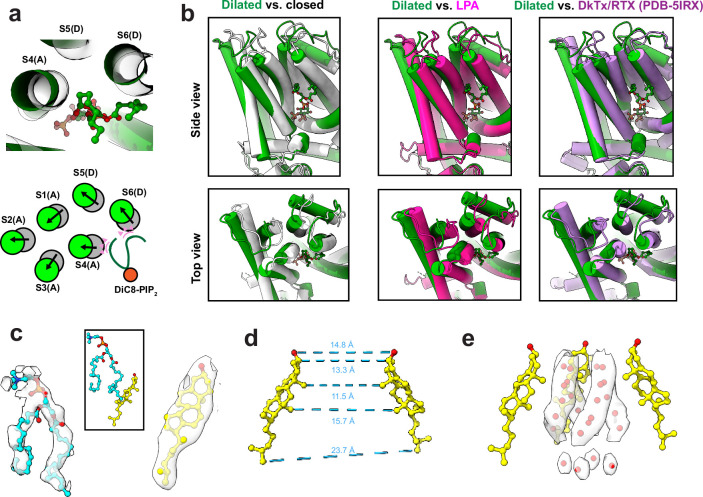
Extended Data Fig. 4. TRPV1 bound with diC8-PIP2 in the dilated state.
(a) Top: Top-down view of diC8-PIP2 (green sticks and balls) and surrounding helices. Grey ghost tubes are TRPV1 bound with diC8-PIP2 in the closed state and green tubes are in the dilated state. Bottom: schematic comparing the helical positions between the closed and dilated states. Pink triangles indicate the steric overlap between the diC8-PIP2 molecule in the dilated state and the transmembrane helices of TRPV1 in the closed state. (b) Comparisons of the dilated state of TRPV1 with diC8-PIP2 bound and other states. (c) Densities for cholesterol and a lipid (modeled as DOPC) that is found in a cleft between the S6 and S5 helices of adjacent monomers (see Fig. 3). (d) Interatom distances between two opposite cholesterol molecules demonstrating the pore radius formed by cholesterol. (e) Extra densities within the pore that are modeled as ordered water molecules due to the hydrophobic effect. 3 of the 4 cholesterol molecules are shown for clarity.