Fig. 2. I-SPY2 clinical trial retrospective analysis.
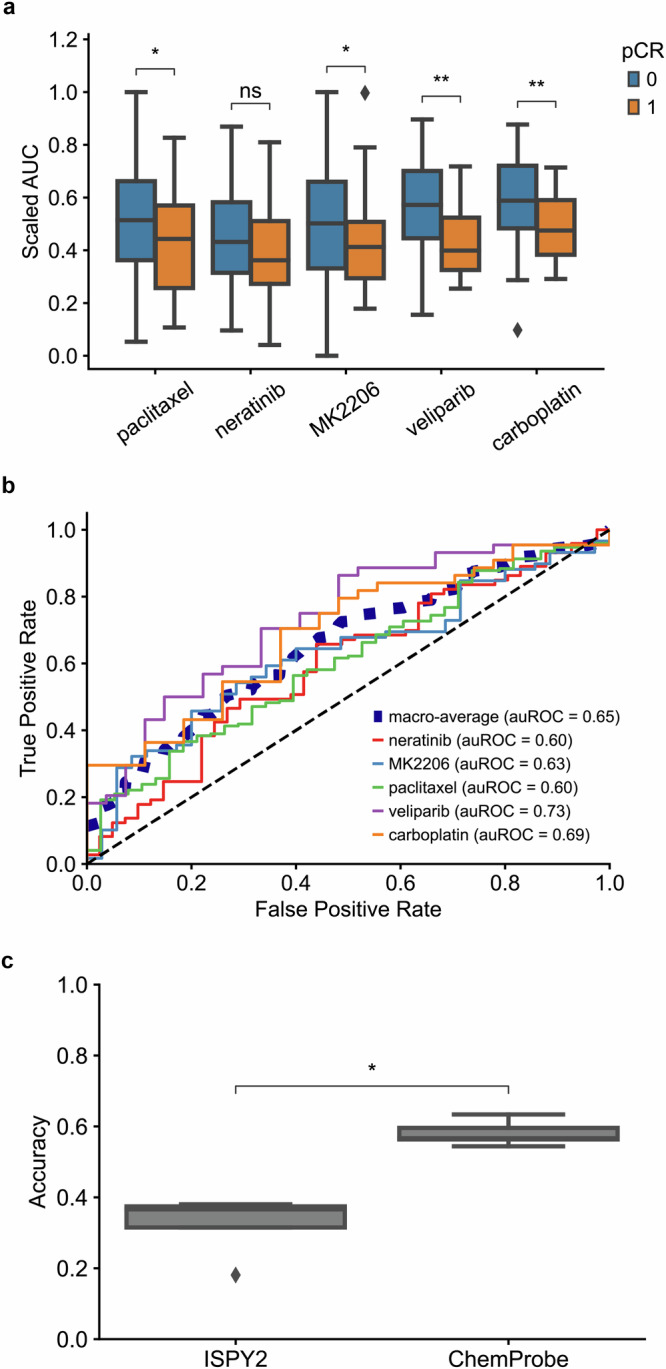
a Predicted dose-response AUC for I-SPY2 patients treated with each drug. AUCs scaled between the minimum and maximum predicted AUC of patients treated with each drug. Blue = non-responder, orange = responder; centerline, median; box limits, upper and lower quartiles; whiskers, 1.5x interquartile range; points, outliers; two-sided Wilcoxon rank-sum test; ns: p < =1e1, *p < =5e-2, **p < =1e-2. Sample sizes: paclitaxel (n = 38 independent case samples, n = 172 independent control samples); neratinib (n = 41 independent case samples, n = 73 independent control samples); MK2206 (n = 35 independent case samples, n = 59 independent control samples); veliparib (n = 27 independent case samples, n = 44 independent control samples); carboplatin (n = 27 independent case samples, n = 44 independent control samples). b Receiver operating characteristic curve of patients treated with each drug and corresponding auROC. c Accuracy of I-SPY2 (n = 5 independent samples) predictions versus ChemProbe (n = 5 independent samples) predictions for non-responders/responders. Centerline, median; box limits, upper and lower quartiles; whiskers, 1.5x interquartile range; points, outliers); two-sided Wilcoxon rank-sum test; *p < =5e-2.
