Abstract
Relict subtropical coniferous forests in China face severe fragmentation, resulting in declining populations, and some are under significant threat from invasive alien species. Despite the crucial importance of understanding forest dynamics, knowledge gaps persist, particularly regarding the impact of invasive plants on vulnerable natives like Keteleeria evelyniana. In this study, we investigated the impact of invasive plants on the regeneration of forests dominated by K. evelyniana, a subtropical relict species in southwestern China. For this purpose, we characterized forest dynamics of 160 forest plots featuring K. evelyniana as the primary dominant species and determined whether the presence of invasive plants was correlated with regeneration of K. evelyniana. We identified four distinct forest types in which K. evelyniana was dominant. We found that radial growth of K. evelyniana trees is faster in younger age-classes today than it was for older trees at the same age. The population structure of K. evelyniana in each forest type exhibited a multimodal age-class distribution. However, three forest types lacked established saplings younger than 10 years old, a situation attributed to the dense coverage of the invasive alien Ageratinaadenophora. This invasive species resulted in a reduction of understory species diversity. Additionally, our analysis uncovered a significant negative correlation in phylogenetic relatedness (net relatedness index) between native and invasive alien plant species in eastern Yunnan. This suggests closely related invasive species face heightened competition, hindering successful invasion. Taken together, our findings indicate that successful establishment and habitat restoration of K. evelyniana seedling/saplings require effective measures to control invasive plants.
Keywords: Keteleeria evelyniana, Age structure, Regeneration, Species diversity, Invasive alien species, Phylogenetic relatedness
Highlights
-
•
Four forest types of the vulnerable relict Keteleeria evelyniana in China are highly fragmented and unprotected.
-
•
The age structure and poor regeneration of K. evelyniana heighten the risks to its populations.
-
•
The invasive alien plant Ageratina adenophora poses a notable risk to K. evelyniana's regeneration and species diversity.
-
•
The observed NRI in eastern Yunnan suggests closely related invasive species may struggle as successful invaders.
-
•
We strongly advocate controlling A. adenophora in K. evelyniana habitats to safeguard the population and forest persistence.
1. Introduction
Relict plants represent the most vulnerable component of a flora. The loss of any relict species marks an irreparable setback for biodiversity as a whole (Tang, 2015; Yesemuratova, 2021). In forest ecosystems, ensuring native tree regeneration and maintaining species diversity can be impeded by invasive alien species, which compete for resources (e.g. space, light, soil nutrients and water) (Wang, 2006; Tang et al., 2011; Poudel et al., 2019; Langmaier and Lapin, 2020), exert phytotoxic effects (Zhou et al., 2013; Rusterholz et al., 2018; Darji et al., 2021), and cause extinctions (Thomas et al., 2004). Thus, it is imperative to elucidate how the community and population structures of forests dominated by relict species are affected by invasive alien species.
Subtropical China, particularly the southwestern region, plays a significant role in the regional biogeography of plants and serves as a long-term refugium for many relict species (Tang et al., 2018). Keteleeria is an ancient relict genus, with fossils dating to the Eocene-Oligocene in Canada, the USA and Japan (Huzita and Kasama, 1983; Meyer and Manchester, 1997; Mathewes et al., 2016), and the Miocene-Pliocene in the USA, China, Japan and Russia (Ozaki, 1981; Liu et al., 1996; Rember, 2010; Blokhina and Bondarenko, 2011). Most species of Keteleeria are now extinct. Keteleeria diverged from its closest genus (Abies) about 60 Mya according to molecular data (Ran et al., 2018). The genus comprises only five extant species, although for two of them (Keteleeria davidiana and K. fortunei) with several varieties are recognized (Fu, 1999). All Keteleeria species are endemic to China, except K. evelyniana, which is distributed in southwestern China, Laos and Vietnam (Yang, 1999). This species has been designated ‘vulnerable’ on the IUCN Red List (Thomas, 2013, 2019), ‘endangered’ in Laos (Averyanov et al., 2014), and placed on the Red Lists of plants in China (Qin et al., 2017) and Vietnam (Nguyen et al., 2007), although recently the Chinese Red List of gymnosperms has designated it as ‘near threatened’ (Yang, 2021). K. evelyniana is recognized as an important component in subtropical coniferous forest ecosystems, for its decorative qualities, and various applications in afforestation, construction, and industry (Yang, 1999; Luu and Thomas, 2004; Zhang et al., 2014). Recent research on K. evelyniana coniferous and broad-leaved mixed forests of Xishan, Kunming, China, have indicated a concerning decline in its population and poor regeneration (Li et al., 2013). Understanding the current state of these relict forests, necessitates comprehensive surveys and analyses encompassing forest characteristics, population structure, and growth trends. However, to date, little is known about K. evelyniana forest types, community and population structures, or growth in the wild.
Invasive alien species have been linked to the extinction of up to 27% of plant species currently listed as extinct in the wild (Bellard et al., 2016). In China, the majority of invasive alien plant species are found in the subtropics (Bai et al., 2013). Ageratina adenophora, one of the most aggressive invasive alien species in subtropical China, influences the structure and function of plant communities and destroys coexisting patterns of species (Chen et al., 2021), as well as causing significant decline of species diversity (Ding et al., 2007). It is unclear whether or how A. adenophora has impacted the regeneration of Keteleeria evelyniana. However, one approach to predicting whether an alien invasion will be successful is to determine the phylogenetic relatedness of invasive alien species and native species (Strauss et al., 2006; Park and Potter, 2013; Yessoufou et al., 2014; Omer et al., 2022; Qian, 2023a, b).
Here, we determined how invasive plant species affect forest ecosystems in which Keteleeria evelyniana, a subtropical relict species from southwestern China, is dominant. We analyzed 160 forest plots across the distribution range of K. evelyniana to identify forest types and characteristics, including growth trends and age structure. We then elucidated the threats posed by invasive alien plants to the regeneration of K. evelyniana and determined the phylogenetic relatedness between native and invasive alien species. We formulate recommendations based on our research for conservation and management of K. evelyniana.
2. Materials and methods
2.1. Study area
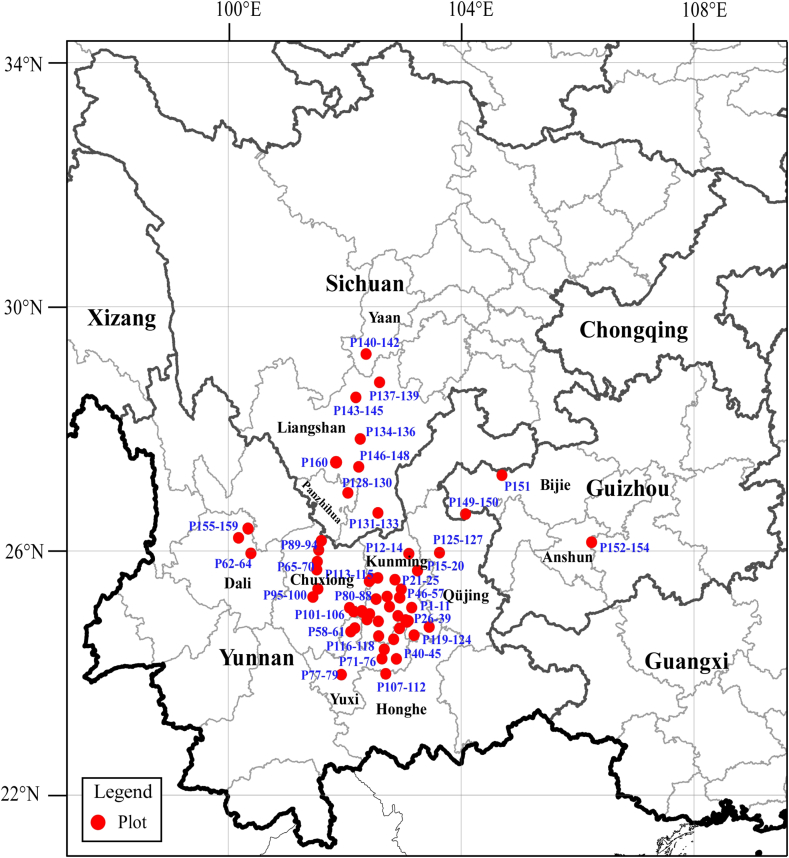
We investigated 160 forest plots (each plot size: 20 m × 20 m; total area of all plots: 64,000 m2) of forests containing Keteleeria evelyniana as the first dominant. These forest plots, which represent the distribution range of K. evelyniana in China, were located in 35 counties, one county-level city (Anning City) of Kunming City, three prefecture-level cities (Yuxi City, Dali City, and Qüjing City), and one prefecture (Chuxiong Prefecture) of Yunnan Province, two prefecture-level cities (Panzhihua City and Yaan City) and one prefecture (Liangshan Prefecture) of Sichuan Province, and two prefecture-level cities (Anshun City and Bijie City) of Guizhou Province, southwestern China (Fig. 1). Detailed information is provided in Appendix 1.
Fig. 1.
Study areas and plot locations in southwestern China.
The study plots ranged from 940 to 2560 m a.s.l. and consisted of red and brown soils. These study plots are generally warm and moist, largely controlled in summer by the Indian Ocean monsoon. The annual mean temperature of the plots ranges between 8.2 and 16.9 °C. The mean temperature of the warmest month, July, is between 13.6 and 22.4 °C and that of the coldest month, January, from 0.8 to 10.2 °C. Annual precipitation is between 793 and 1284.5 mm, and the evapotranspiration is 563.8–832.3 mm. The moisture index (the ratio of mean annual actual evapotranspiration to mean annual potential evapotranspiration) is 0.8–1.0. Additional information on environmental characteristics of plots is shown in Table S1.
2.2. Study species
Keteleeria evelyniana is an evergreen coniferous canopy tree with a crown that can become broad as the tree ages (Carrière, 2021). It can reach 40 m tall and 100 cm in diameter at breast height (DBH). The leaves are flat, needle-like. Compared to K. davidiana and K. fortunei, which are very prickly and can spike when grabbed, the foliage of K. evelyniana is soft and not unpleasant when touched. It has ability to sprout from a stump (Creech, 2016). Seed cones are cylindrical. Seeds are oblong with yellowish brown wings. Seed maturity is in October (Yang, 1999). It has thick phellem layer, with strong fire resistance. Wood is usually tan or hazel. The wood dries quickly, the deformation is small, the specific gravity is light, and it is easy to process. It is generally considered to be better quality than that of Pinus spp. (Fern, 2022).
Ageratina adenophora (Asteraceae), also called Crofton weed, is a perennial herb or subshrub native to Mexico (Auld, 1970). It grows to 200 cm tall. The small compound flowers occur in late spring and summer, and are found in clusters at the end of branches. Each small flower is followed by a small brown seed with a white feathery ‘parachute’ that can be dispersed by the wind similar to a dandelion (Wolff, 1999). A mature plant produces 10,000–100,000 seeds per year, which can be transported by wind over very long distances due to their very low weight (Gosper, 2003). It is poisonous, oxen and horses have become ill and have sometimes died from consuming it (Wang, 2005).
2.3. Data collection and analyses
2.3.1. Forest structure and species diversity
Forest plots included various types of Keteleeria evelyniana-dominated plant communities were established (Fig. S1). All data were collected in January, May and September of 2020 and 2021. We divided each plot into subplots. The size of each subplot was 10 m × 10 m. For the species in each plot, all individuals at least 1.3 m tall were identified to species level, numbered and tagged, and their diameter at breast height (DBH; 1.3 m tall) and height were recorded. We included K. evelyniana trees with a DBH > 65 cm outside but near a plot. In addition, general information about each plot was annotated, such as slope position, elevation, slope exposure, slope inclination, as well as human disturbance history. Woody stems (≥ 1.3 m tall) in the overstory were classified into two categories based on their vertical position and height: arborous layer (height ≥ 5 m) and shrub layer (1.3 m ≤ height < 5 m tall) (Tang et al., 2022). The arborous layer included emergent (height > 20 m), canopy (10 m ≤ height ≤ 20 m), and subcanopy (5 m ≤ height < 10 m) sublayers. For all woody species less than 1.3 m tall in the understory, each individual was identified to species level, counted, and measured for height and percent cover. In each subplot, we set up five 1 m × 1 m squares to investigate the woody and herbaceous taxa in the understory, which were located in the four corners and the center of the subplot. The taxa in the understory were identified and the coverage, height, and number of individuals of each species were recorded. Individuals of K. evelyniana with a height between 30 and 130 cm were considered established seedlings. We additionally surveyed and recorded the coverage of each invasive alien species in the whole understory of each plot. To identify which of the observed species were invasive alien species, we used the most recent accounts on invasive alien species for China (Hao and Ma, 2023).
The intensity of human disturbance of each plot was evaluated as follows. (1) No/slight intensity refers to no disturbance, untouched or nearly untouched forest. (2) Medium intensity refers to one of the two treatments namely selective cutting harvesting for fuelwood or road construction close to the forests. (3) Severe intensity refers to more than two disturbances.
For all woody individuals ≥ 1.3 m tall, DBH was used to calculate basal area (BA) and then basal area for each species found in a plot could be determined. To measure the abundance of species, we used relative importance value (RIV) = (Relative density + Relative basal area)/2 for species in the overstory, and RIV = (Relative density + Relative coverage)/2 for species in the understory (Tang et al., 2022). Plant communities were classified using a floristic similarity dendrogram (PCORD software; McCune and Mefford, 2016). The communities were named by dominant/indicator species of the overstory. The dominant species in either the overstory or understory were defined by relative importance values. Diversity was calculated for forest understory using species richness (number of species), Shannon–Wiener's diversity index H′ (Pielou, 1969) and Simpson's diversity index (Lander, 1996).
2.3.2. Tree core analysis of Keteleeria evelyniana
We obtained 49 increment cores from Keteleeria evelyniana trees of varying DBHs in the study area. For each tree trunk, a single increment core was taken at 1.3 m above ground level. According to tree rings in the stem base of saplings with a height ≤ 1.3 m, trees took about eight years to grow 1.3 m in height. The eight years was added to the data of ages we obtained from each increment core. In addition, because it is difficult to use increment borers to get increment cores from K. evelyniana trees with DBHs ≤ 10 cm, we selected 17 young trees with DBHs ≤ 10 cm and cut the stem at exactly 1.3 m above the ground level for each young tree to obtain its cross section for age based on tree rings. Tree age was determined using the software WinDENRO tree ring analysis system (Regent Instruments Inc., Canada).
We used 66 tree samples to determine the correlation of ages and DBHs. Then we used the slope of the line to calculate ages according to the measured DBHs of Keteleeria evelyniana trees in the study plots. From tree ring analysis, we were also able to determine ring widths and to calculate basal area increments (BAI). The following formula was used to calculate BAI: T − Y, where T is the basal area at year X (last year of growth) and Y is the basal area of the tree measured up to the year previous to X. BAI is used in forest growth studies because it accurately quantifies wood production based on the ever-increasing diameter of a growing tree (Rubino and McCarthy, 2000).
2.3.3. Phylogenetic analysis and phylogenetic relatedness
To explore the phylogenetic structure of Keteleeria evelyniana forests in China, we constructed a phylogenetic tree of all 338 species identified (see below) in our studied K. evelyniana forest plots. We obtained a list of all the species from the data of our vegetation plots in the field, and proofread their accepted names from the ‘’WFO Plant List (https://wfoplantlist.org/taxon/). According to the list, we generated a phylogenetic tree based on the time-calibrated phylogeny of Smith and Brown (2018) by the package ‘V.PhyloMaker’ (Jin and Qian, 2019, 2022) in R (v.4.1.0; R Core Team, 2021).
Two commonly used metrics of phylogenetic relatedness, net relatedness index (NRI) and nearest taxon index (NTI), are widely used in community phylogenetic analyses. NRI is based on the mean phylogenetic distance (MPD) between all species pairs across a given phylogeny, while NTI is derived from the mean nearest taxon distance (MNTD), i.e., the average phylogenetic distance of each species from its closest relative. Thus, NRI and NTI tend to respectively reflect phylogenetic relatedness at deep (i.e., phylogeny-wide) and shallow (i.e., across the phylogeny tips) evolutionary history. Positive NRI and NTI both indicate phylogenetic clustering, which is the expected result of habitat filtering (i.e., closely related species are more similar in their ecological requirements and, thus, are more likely to coexist). In contrast, negative values of both NRI and NTI imply over-dispersion, meaning closely related species are less likely to coexist, which is due to competitive exclusion (Webb et al., 2002; Swenson, 2009). We analyzed the differences in phylogenetic indices among each forest type by calculating NRI and NTI for all species, utilizing the R package ‘picante’ (v.1.8.2; Kembel et al., 2010), followed by conducting descriptive statistical analysis and creating a boxplot using R (v.4.1.0; R Core Team, 2021).
Furthermore, we explored the phylogenetic relatedness between native and invasive alien species of the understory of Keteleeria evelyniana forests. We classified the study areas where invasive alien species were found as four areas, i.e., central Yunnan, south-central Yunnan, eastern Yunnan and southwestern Sichuan. We calculated NRI and NTI for phylogenetic relatedness between native and invasive alien plant species of the forest understory in the four areas as a whole and in each of the four areas. Then, we assessed correlations in phylogenetic relatedness metrics (NRI, NTI) between native and invasive alien plant species using cor.test function of stats package in R (v.4.1.0; R Core Team, 2021).
Relationships between variables were statistically tested with Pearson's correlation coefficient as well as a p value using R (v.4.1.0; R Core Team, 2021).
3. Results
3.1. Forest types and characteristics
The 160 vegetation plots of our study area exhibited floristic features of forests with tropical–especially subtropical/warm temperate (the transitional from tropical to temperate) –affinities. A total of 338 plant species in 239 genera and 100 families were recorded (Table S2). Of these, 31% of families and 23% of genera were tropical; 21% of families and 20% of genera were temperate. We recorded 20 invasive alien species, with Ageratina adenophora, an indicator of disturbed habitats, dominant in the forest understory at many sites.
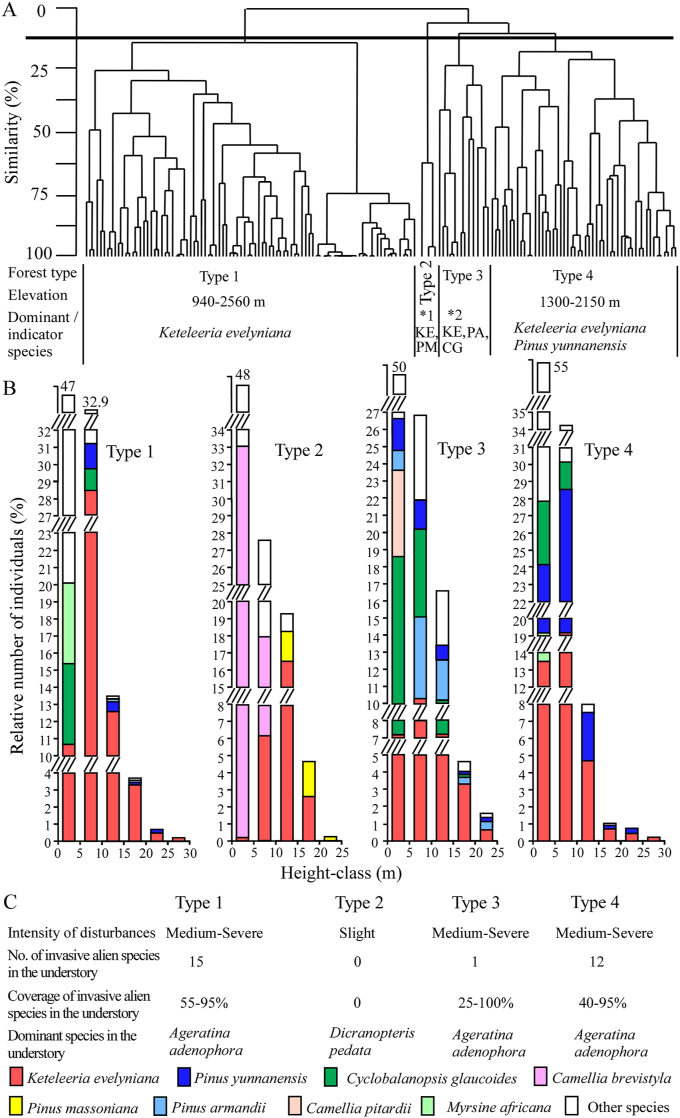
Floristic similarity cluster analysis of our study sites identified four distinct forest communities (at the 13% floristic similarity threshold) (Fig. 2A): Keteleeria evelyniana evergreen coniferous forest (Type 1); K. evelyniana–Pinus massoniana evergreen coniferous forest (Type 2); K. evelyniana–Pinus armandii–Cyclobalanopsis glaucoides evergreen coniferous and broad-leaved mixed forest (Type 3); K. evelyniana–Pinus yunnanensis evergreen coniferous forest (Type 4).
Fig. 2.
Cluster analysis and the frequency distribution in height-classes of woody species and habitat characteristics. (A) Cluster analysis of the 160 plots. (B) The frequency distribution in height-classes of woody species (height ≥ 1.3 m) of each forest type. (C) The intensity of disturbances, the number and coverage of invasive alien species in the understory of each forest type. KE: Keteleeria evelyniana; PM: Pinus massoniana; PA: Pinus armandii; CG: Cyclobalanopsis glaucoides; ∗1: 1200–1300 m; ∗2: 1700–2300 m; Type 1: Keteleeria evelyniana evergreen coniferous forest; Type 2: Keteleeria evelyniana–Pinus massoniana evergreen coniferous forest; Type 3: Keteleeria evelyniana–Pinus armandii–Cyclobalanopsis glaucoides evergreen coniferous and broad-leaved mixed forest; Type 4: Keteleeria evelyniana–Pinus yunnanensis evergreen coniferous forest.
For the forest's arborous and shrub layers, the vertical stratification for woody species (height ≥ 1.3 m) is depicted in Fig. 2B. The intensity of disturbances, the number and coverage of invasive alien species in the understory are shown in Fig. 2C.
Keteleeria evelyniana evergreen coniferous forest (Type 1) were located on gentle or steep slopes at elevations between 940 and 2560 m in central, eastern and northwestern Yunnan, southwestern Sichuan, northwestern and west-central Guizhou. K. evelyniana dominated (relative importance value (RIV) = 90%) the canopy (height: 10–20 m) and subcanopy (height: 5–10 m) of these forests, and reached 25 m tall in the emergent sublayer (Fig. 2B and Table S3). Few tree species (e.g., Pinus yunnanensis and Quercus acutissima) were found in the canopy and subcanopy. K. evelyniana also dominated the shrub layer, which included Cyclobalanopsis glaucoides, Myrsine africana, etc (Table S4). In the understory, 15 invasive alien species were found (Fig. 2C), although Ageratina adenophora was dominant. Other invasive alien species included Bidens pilosa, Galinsoga parviflora, Lantana camara, and Praxelis clematidea, etc (Table S5).
Keteleeria evelyniana–Pinus massoniana evergreen coniferous forests (Type 2) grew on upper slopes at elevations of 1200–1300 m in northwestern Guizhou. K. evelyniana dominated (RIV = 66%) the canopy (10–20 m) and subcanopy (5–10 m), and reached 17 m tall (Fig. 2B and Table S3). Pinus massoniana was co-dominant RIV = 20%) in the canopy and reaches 24 m in the emergent sublayer. The subcanopy also contained trees of Myrica rubra, Quercus aliena and Corylus heterophylla var. sutchuenensis, among others. The shrub layer was dominated and densely covered by the shrub Camellia brevistyla, accompanied by Viburnum erosum, M. rubra, and Q. aliena var. aliena (Table S4). In the understory, the herb Dicranopteris pedata and the seedlings of C. brevistyla were co-dominant. Other herbs frequently observed included Miscanthus floridulus and Pteris cretica, etc (Table S5). Invasive alien plants were absent in these forests (Fig. 2C).
Keteleeria evelyniana–Pinus armandii–Cyclobalanopsis glaucoides evergreen coniferous and broad-leaved mixed forest (Type 3) grew in valley bottoms or mountain slopes at elevations of 1700–2300 m in central and northwestern Yunnan. Few trees of K. evelyniana reached 24 m tall in the emergent sublayer. K. evelyniana (RIV = 53%), P. armandii (RIV = 13%), and C. glaucoides (RIV = 11%) co-dominated the canopy and subcanopy (Fig. 2B and Table S3). Trees of other species, such as Pinus yunnanensis, Quercus acutissima and Castanopsis delavayi, were also present in this layer. The shrub layer was mainly composed of saplings of canopy trees of K. evelyniana and C. glaucoides, and the shrubs Camellia pitardii, Ternstroemia gymnanthera, Vaccinium bracteatum, etc (Table S4). In the understory, only one invasive alien species (A. adenophora) was found (Fig. 2C), which was dominant and occurred along with native plants of Pteris cretica, Oplismenus undulatifolius, etc (Table S5).
Keteleeria evelyniana–Pinus yunnanensis evergreen coniferous forest (Type 4) grew on gentle slopes or steep slopes at elevations of 1300–2150 m in central Yunnan, southwestern Sichuan and northwestern Guizhou. Few K. evelyniana trees reached 25 m tall in the emergent sublayer, while P. yunnanensis reached 24 m tall. K. evelyniana (RIV = 59%) and P. yunnanensis (RIV = 29%) co-dominated the canopy. K. evelyniana occupied the subcanopy, with accompanying species mainly including Q. acutissima and Cyclobalanopsis glaucoides (Fig. 2B and Table S3). In the shrub layer, there were mainly saplings of K. evelyniana and P. yunnanensis, accompanied by shrubs including M. africana, Pyrus pashia, etc (Table S4). In the understory, 12 invasive alien species were found (Fig. 2C). A. adenophora was dominant, with other invasive alien species also found (e.g., Bidens pilosa, Chamaecrista mimosoides, Galinsoga parviflora, and Phytolacca americana) (Table S5).
All invasive alien plant species and their coverages are summarized in Table S6.
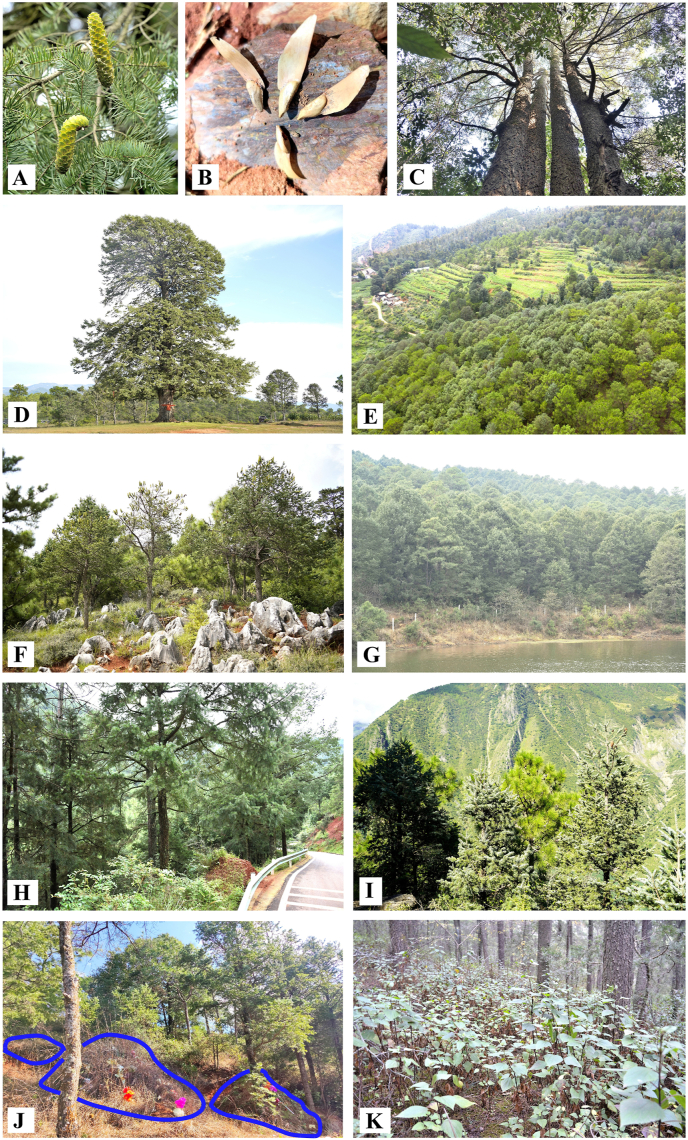
All forest types except the Keteleeria evelyniana–Pinus massoniana evergreen coniferous forest have experienced medium to severe human disturbance (Fig. 2C). All four forest types were fragmented due to the discontinuous distribution of patchy forests. They were commonly surrounded by secondary evergreen broad-leaved forests, secondary Pinus forests, agriculture fields, roadsides, or situated near plateau lakes. Notably, none of these forests have been situated within protected areas, such as nature reserves. Fig. 3A–K illustrates representative forest stands of K. evelyniana and their habitats.
Fig. 3.
Keteleeria evelyniana and its representative forest stands and habitats. (A) Seed corns and foliage of K. evelyniana; (B) Winged seeds of K. evelyniana; (C) The canopy of a K. evelyniana forest at 1988 m a.s.l. in Longshan, Wenquan Zhen, Anning City, Yunnan Province; (D) A 208-year-old K. evelyniana tree, ca. 146 cm DBH, 27 m tall at 2560 m a.s.l in Sankeshu Village, Wu Town, Yanyuan County, Sichuan Province; (E) A fragmented forest of K. evelyniana surrounded by farmland at 2044 m a.s.l. in Tanglangqing, Luquan County, Yunnan Province; (F) A fragmented forest of K. evelyniana at 1730 m a.s.l. in the Karst area of Sijiaotian Village, Shilin County, Yunnan Province; (G) A fragmented forest of K. evelyniana by a lake at 1813 m a.s.l. in Shuangmei Village, Anning City, Yunnan Province; (H) A fragmented forest of K. evelyniana by a road at 1215 m a.s.l. in Shuchang, Meiqi Village, Anshun City, Guizhou Province; (I) A fragmented forest of K. evelyniana at 2100 m a.s.l. in Mulihe, Bajia Village, Muli County, Sichuan Province; (J) The forest floor was used for burial plots (indicated by the blue lines) by local villagers at 1990 m a.s.l. in Chenggong District, Kunming, Yunnan Province; (K) An invasive alien species, Ageratina adenophora, densely occupies the understory of a K. evelyniana forest at 2020 m a.s.l. in Danaobao, Jiulongsi Village, Yiliang County, Yunnan Province.
3.2. Population characteristics
3.2.1. Growth trends
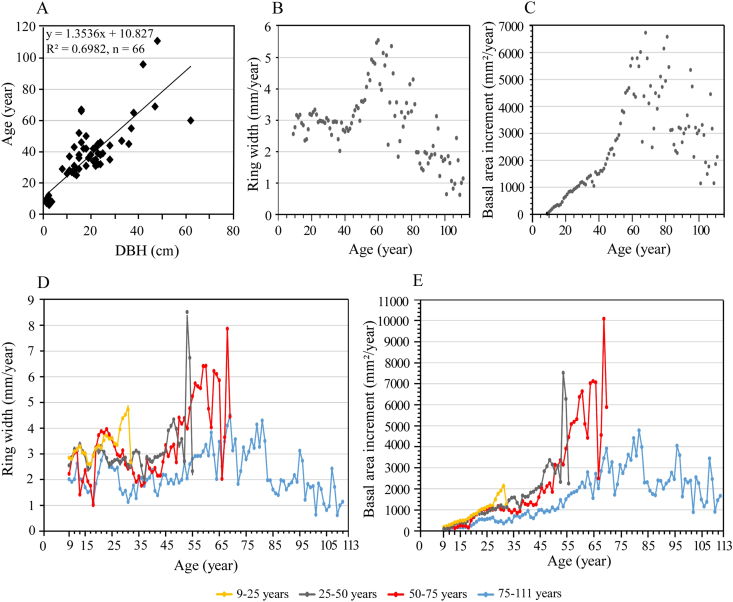
For Keteleeria evelyniana, age and DBH were positively correlated (y = 1.3536x + 10.827, R2 = 0.6982, n = 66) (Fig. 4A; p < 0.05), which allowed us to use DBH to determine the age of each K. evelyniana individual.
Fig. 4.
Growth trends of Keteleeria evelyniana trees (height ≥ 1.3 m). (A) Relationships of DBH and age; (B) Changes in ring width over the years; (C) Changes in basal area increment over the years; (D) Ring widths in the four age classes (i.e., 9–25, 25–50, 50–75, 75–111 years) over the years; (E) The basal area increment in the four age classes over the years.
Ring width of Keteleeria evelyniana (n = 49 individuals) indicated that seedlings generally required 8 years to reach 1.3 m in height. The ring width growth rate gradually increased from 2 to 3.1 mm/year (average 2.9 mm/year) between 9 and 45 years of age, peaking at 5.6 mm/year at 60 years, and then declining to 0.6 mm/year at 108 years (Fig. 4B). Basal area increment (BAI) increased gradually up to 40 years of age, followed by a rapid rise from 1214.8 to 6621.3 mm2/year at 65 years. BAI generally decreased, albeit with fluctuations, post-60 years (Fig. 4C).
Both ring width and basal area increment indicated that Keteleeria evelyniana trees aged 9–25 years were growing at a higher rate than trees aged 25–50 years at the same age. This pattern was observed across subsequent age intervals. Thus, the fastest growth rates occurred in 1996–2021, followed by 1971–1996, and 1946–1971. The slowest growth rates occurred from 1910 to 1946 (Fig. 4D and E).
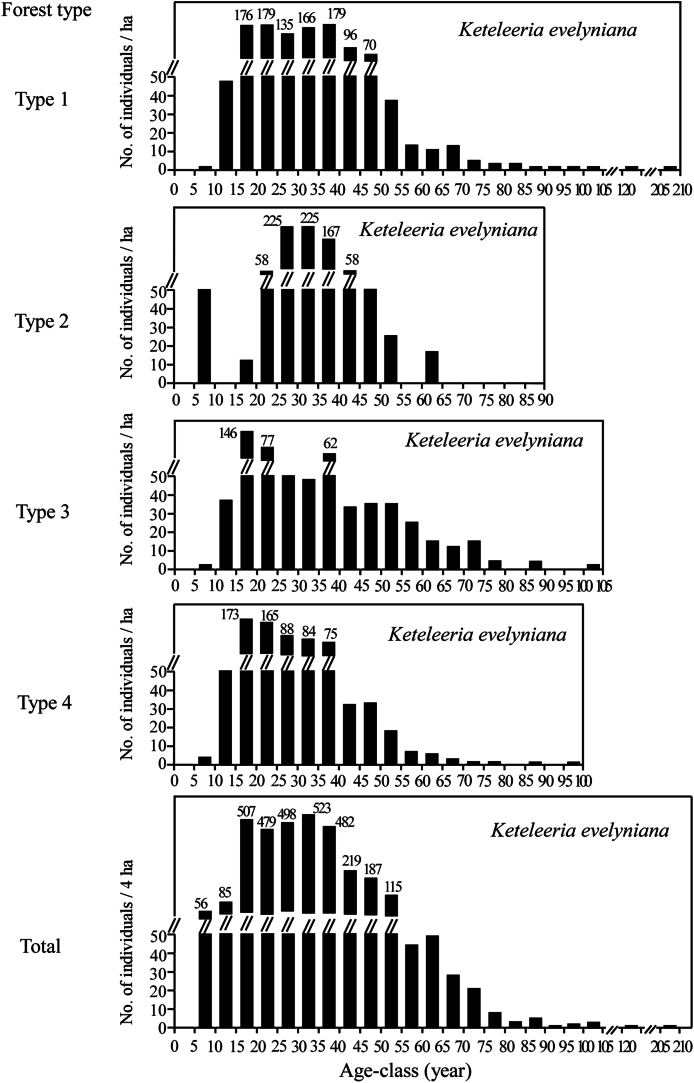
3.2.2. Age structure
The age structure of Keteleeria evelyniana for each forest type reveals a multimodal distribution (Fig. 5). The observed maximum age reached 208 years in forest Type 1 and 65 years in Type 2, while in Types 3 and 4 it was 105 and 100 years, respectively. Few trees fell within the age range of 75–208 years. The number of trees aged 10–15 years was notably low, and there were scarce to no established saplings under 10 years old, except in forest Type 2.
Fig. 5.
The age structure of Keteleeria evelyniana. Type 1: Keteleeria evelyniana evergreen coniferous forest; Type 2: Keteleeria evelyniana–Pinus massoniana evergreen coniferous forest; Type 3: Keteleeria evelyniana–Pinus armandii–Cyclobalanopsis glaucoides evergreen coniferous and broad-leaved mixed forest; Type 4: Keteleeria evelyniana–Pinus yunnanensis evergreen coniferous forest.
3.3. Impacts of invasive alien species on regeneration and understory species diversity
Keteleeria evelyniana seedlings are shade-intolerant. At the initial stage of seedling growth (0–30 cm tall), K. evelyniana individuals were observed at high density beneath the forest canopy, likely due to seed dispersal mainly around parent trees. Over time, however, few surviving seedlings/saplings (with a height of 90–130 cm) survived, and were mainly found in open micro-habitats, i.e. forest gaps (Fig. S2).
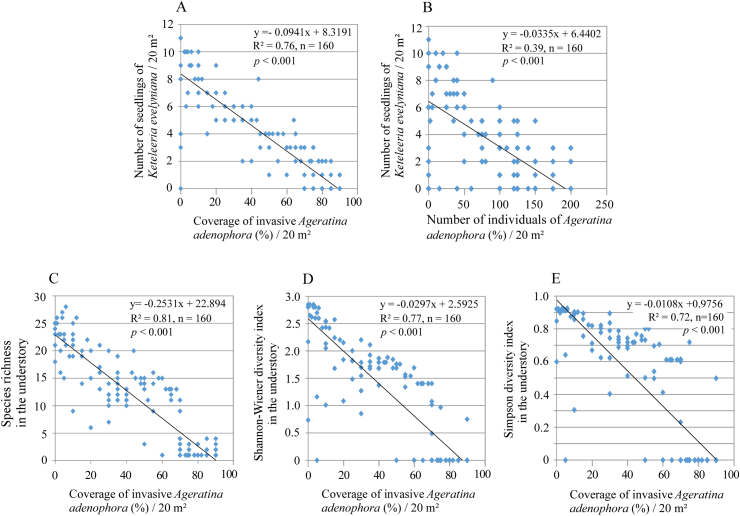
Seedling recruitment and establishment were hindered by invasive alien species, especially Ageratina adenophora. The number of seedlings of Keteleeria evelyniana were negatively correlated with coverage (R2 = 0.76, n = 160, p < 0.001; Fig. 6A), and with density (R2 = 0.39, n = 160, p < 0.001; Fig. 6B) of A. adenophora. In addition, species richness and species diversity indices for the understory were significantly reduced by the coverage of A. adenophora (p < 0.001) (Fig. 6C–E).
Fig. 6.
Negative impacts of invasive alien species on number of seedlings of Keteleeria evelyniana and understory species diversity. (A) & (B) Changes in numbers of seedlings of Keteleeria evelyniana by respective coverage and density of invasive alien Ageratina adenophora; (C), (D) & (E) Changes of species richness, Shannon–Wiener diversity and Simpson diversity, respectively, in the understory by coverage of invasive alien Ageratina adenophora.
3.4. Phylogenetic relatedness in Keteleeria evelyniana forests
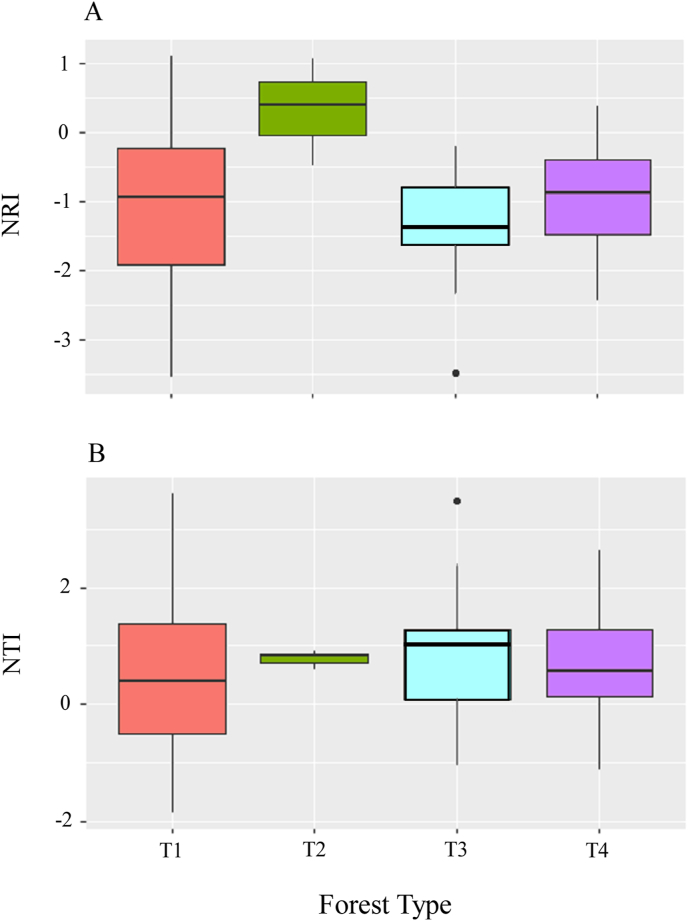
Phylogenetic relatedness among the four forest types indicated over-dispersion for Keteleeria evelyniana evergreen coniferous forest (Type 1) (NRI = −0.93), K. evelyniana–Pinus armandii–Cyclobalanopsis glaucoides evergreen coniferous and broad-leaved mixed forest (Type 3) (NRI = −1.38), and K. evelyniana−Pinus yunnanensis evergreen coniferous forest (Type 4) (NRI = -0.86) (Fig. 7A). In contrast, our analysis indicated that K. evelyniana–Pinus massoniana evergreen coniferous forest (Type 2) was phylogenetically clustered (NRI = 0.39). Among all the forest types, only forest Type 2 and Type 3 differed significantly (p < 0.05, Table 1). However, NTI values indicated that all four forest types were phylogenetically clustered (median values from 0.4 to 1.02) (Fig. 7B), and forests were not significantly different (Table 1).
Fig. 7.
Phylogenetic indices (NRI, NTI) of each forest type. NRI: Net relatedness index; NTI: Nearest taxon index; T1 (Type 1): Keteleeria evelyniana evergreen coniferous forest; T2 (Type 2): Keteleeria evelyniana–Pinus massoniana evergreen coniferous forest; T3 (Type 3): Keteleeria evelyniana–Pinus armandii–Cyclobalanopsis glaucoides evergreen coniferous and broad-leaved mixed forest; T4 (Type 4): Keteleeria evelyniana–Pinus yunnanensis evergreen coniferous forest.
Table 1.
The significant difference among forest types in phylogenetic relatedness metrics (NRI, NTI). NRI: Net relatedness index; NTI: Nearest taxon index; T1 (Type 1): Keteleeria evelyniana evergreen coniferous forest; T2 (Type 2): Keteleeria evelyniana–Pinus massoniana evergreen coniferous forest; T3 (Type 3): Keteleeria evelyniana–Pinus armandii–Cyclobalanopsis glaucoides evergreen coniferous and broad-leaved mixed forest; T4 (Type 4): Keteleeria evelyniana–Pinus yunnanensis evergreen coniferous forest.
| Forest types | NRI (p) | NTI (p) |
|---|---|---|
| T2–T1 | 0.06 | 0.968 |
| T3–T1 | 0.695 | 0.731 |
| T4–T1 | 0.768 | 0.854 |
| T3–T2 | 0.028∗ | 0.999 |
| T4–T2 | 0.126 | 0.996 |
| T4–T3 | 0.39 | 0.95 |
NRI of the understory of the forest plots in all areas as a whole indicated that native and invasive alien species were phylogenetically distinct, although this relationship was weak and not statistically significant (r = −0.277, p = 0.056). When we analyzed phylogenetic relatedness among central Yunnan, south-central Yunnan, eastern Yunnan, and southwestern Sichuan, we found that native and invasive species were phylogenetically distinct in plots of eastern Yunnan (r = −0.999, p < 0.01) (Table 2). NTI did not reveal significant correlations between native and invasive species in plots of all the areas, both individually or as a whole (Table 2).
Table 2.
Pearson correlation coefficients (r) of comparisons in phylogenetic relatedness metrics (NRI, NTI) between native and invasive alien plant species of the four geographic areas as a whole and each area. NRI: Net relatedness index; NTI: Nearest taxon index.
| Geographic areas for forest plots | NRI |
NTI |
||
|---|---|---|---|---|
| r | p | r | p | |
| All areas | −0.277 | 0.056 | −0.02 | 0.89 |
| Central Yunnan | −0.132 | 0.602 | 0.356 | 0.148 |
| Southcentral Yunnan | −0.599 | 0.089 | −0.467 | 0.205 |
| Eastern Yunnan | −0.999 | 0.004* | −0.954 | 0.195 |
| Southwestern Sichuan | 0.006 | 0.984 | −0.109 | 0.723 |
4. Discussion
4.1. Forest characteristics
Keteleeria evelyniana forest communities, which are distributed between 940 and 2560 m a.s.l., fall within the subtropical evergreen broad-leaved forest zone in Yunnan, Sichuan and Guizhou Provinces. These forests are located near roadsides, farmland and plateau lakes, in valley bottoms, gentle slopes, steep slopes, and limestone habitats. The most important canopy trees associated with K. evelyniana are the coniferous Pinus yunnanensis, P. armandii, and P. massoniana, and the broad-leaved Cyclobalanopsis glaucoides and Quercus acutissima. Shrub associates include Myrsine africana, Ternstroemia gymnanthera, Camellia pitardii, Vaccinium bracteatum, and Camellia brevistyla, among others. The understories of all forest types mainly consist of the invasive alien plant Ageratina adenophora, except for K. evelyniana–Pinus massoniana evergreen coniferous forests, where the shrub layer is dense with C. brevistyla. All K. evelyniana forests examined are highly fragmented and appear as patchy mosaics due to human activities.
The species found in Keteleeria evelyniana forests of China differ from those found in Laos and Vietnam, as they are associated with different coniferous species, namely Cephalotaxus mannii, Dacrycarpus imbricatus, Dacrydium elatum, Nageia wallichiana, Pinus dalatensis, Pinus latteri, Pinus kesiya, and Podocarpus neriifolius (Averyanov et al., 2014; Loc et al., 2017). In Vietnam, K. evelyniana forests have been greatly reduced and fragmented by forest conversion (Hiep et al., 2004).
Although Keteleeria evelyniana and K. davidiana forests are both subtropical forests that have a partially sympatric distribution in China, these forests differ in species composition and elevational range. Forest communities of K. davidiana var. davidiana are scattered in the subtropical evergreen broad-leaved forest zone between 100 and 1800 m a.s.l. in the hills, mountains, and hot and dry valleys of Hubei, Guangxi and Yunnan Provinces. The canopy trees of K. davidiana var. davidiana forests include Quercus acutissima, Q. variabilis, Carpinus cordata, Platycarya strobilacea, Pinus yunnanensis, and Pinus massoniana. Shrub associates consist of Cotinus coggygria var. pubescens, Loropetalum chinense, Rhododendron simsii, and Lyonia ovalifolia, among others (Zhao, 2009; Bai et al., 2017; He et al., 2018; Chuan et al., 2021). In the forest communities of Hubei, the understory either does not contain the invasive alien Ageratina adenophora or this species is not a dominant component of the understory. One explanation for these differences is that K. davidiana var. davidiana communities are located in nature reserves that lack human disturbances. In south-central Yunnan, there is a small K. davidiana var. davidiana population that is declining due to human activity and habitat destruction (Chuan et al., 2021).
Keteleeria davidiana var. formosana is scattered in evergreen broad-leaved forests at low elevations at the northern tip of Taiwan (300–600 m a.s.l.), and in southern Taiwan (500–900 m a.s.l.) (Li, 1975). There are 200 plants (mostly old mature plants and with little signs of any natural regeneration) of this variety in the Pinglin Nature Reserve in Taiwan. A subpopulation in Taitung County comprises 500 mature individuals (average DBH up to 80 cm). Only two seedlings have been found within this subpopulation (Luscombe et al., 2019).
4.2. Growth trends
In the forests we investigated, the ring width growth rate of Keteleeria evelyniana trees is lower (2.95 mm/year on average within first 30 years) than that of congeneric species K. davidiana var. davidiana (5.7 mm/year on average for first 30 years, recalculated from Wei et al., 2014) in its natural secondary forest in Yachang Linchang, Baise, northwestern Guangxi. Interspecific differences in tree growth have been often reported even in locations where congeners co-occur (e.g., Quercus prinus vs. Quercus rubra; Rollinson et al., 2016). In fact, in addition to intrinsic factors that are specific to each species (e.g., genetic diversity), ring width growth depends on a wide range of abiotic variables (e.g., temperature, availability of water, light or nutrients, or pests). Thus, intraspecific differences can also be large, even in the same location if there are subtle differences in abiotic factors; for example, site exposure has been shown to produce variation in ring width data in individuals of Pinus heldreichii separated by less than 1 km in the Pindus Mountains of Greece (Klippel et al., 2017). An additional factor that produces higher ring width growth rates is horticultural care (e.g., artificial irrigation, adequate spacing, pruning, and fertilization) (Zahner et al., 1964; Terral and Arnold-Simard, 1966; Rogling et al., 2003; Terral and Durand, 2006). Thus, we are unsurprised that we found the ring width growth rate (2.8 mm/year on average) within the first 17 years of K. evelyniana is lower than that of planted individuals (9.45 mm/year on average for first 17 years; recalculated from Liu et al., 2016).
We found that Keteleeria evelyniana trees in younger age-classes generally grew faster than older trees at the same age; i.e., the closer to the present, the faster the ring growth. Several recent studies have indicated that these types of tree growth patterns may be associated with global warming (Salzer et al., 2009; McMahon et al., 2010; Ning et al., 2022). Specifically, researchers have proposed that higher temperatures over the growing season increase metabolic rates; earlier springs produce longer growing seasons; and higher atmospheric CO2 levels increase photosynthesis (McMahon et al., 2010). Most of these explanations describe trees growing at high elevations and/or high latitudes, where tree growth is limited by (low) temperature but not water availability. In areas where climate change increases drought, tree growth generally declines (Sarris et al., 2007; Thabeet et al., 2009; Keyimu et al., 2020). For example, drought-prone Mediterranean populations of Abies alba showed growth decline during recent decades, whereas populations located further displayed radial growth during the same period (Gazol et al., 2015). However, many studies have shown that the response of tree growth to climate is often species-specific. In northeastern China, climate warming and its associated droughts increased ring growth of some tree species, while it reduced ring growth in other tree species (Yuan et al., 2021a).
It is important to note that the direct impact of plant invasion in the understory on the growth patterns of Keteleeria evelyniana in the overstory remains unclear, as growth was measured after seedling establishment, when K. evelyniana individuals were at least 1.3 m, which typically corresponds to 8 years old. To date, no studies have investigated the influence of plant invasion on growth patterns of K. evelyniana.
4.3. Regeneration of Keteleeria evelyniana, understory species diversity, and phylogenetic relatedness between native and invasive alien species
Invasive alien plant species are capable of altering species composition and species richness of the ground vegetation (Ding et al., 2007; Chen et al., 2021). Understanding the processes affecting invasion dynamics is crucial for promoting the successful regeneration of native tree species (Langmaier and Lapin, 2020). Disturbances and forest fragmentation favor the spread of invasive alien species in forest ecosystems by increasing light and nutrient availability (Raghubanshi and Tripathi, 2009; Martin et al., 2009; González-Moreno et al., 2013). In the heavily disturbed habitats under study, up to 20 invasive alien plant species are found, especially Ageratina adenophora which invades aggressively in the forest understory (it generally dominates the understory with coverages of 25%–90%; Table S6). Together with this species, the vast majority of the invasive alien plants detected by us are of American origin, mostly belonging to Asteraceae or Solanaceae, a common pattern detected in Yunnan Province (Zhang et al., 2018) and throughout China (Zhang et al., 2021; Lin et al., 2022). Studies have indicated that Yunnan is one of the hotspots of plant invasions in China (Chen et al., 2021). Yunnan is the Chinese province with the highest number of invasive alien plant species (Yan et al., 2014; Lin et al., 2022; Hao and Ma, 2023). Certainly, Yunnan has often acted as a gate for plant invasions at national level, with many first detections, including some species we observed here, e.g., A. adenophora, Crassocephalum rubens, Galinsoga parviflora, and Salvia tiliifolia (Xu et al., 2012; Hu et al., 2013).
Ageratina adenophora, which originated from Mexico, is a destructive invasive alien species in China (Wang and Wang, 2006; Lin et al., 2022). A. adenophora first invaded southern Yunnan from Myanmar in the 1940s (Xie et al., 2001; Wang, 2006; Sang et al., 2010), and after a lag phase of 20 years (1940–1960), it has been spreading northeast. Most forest plots studied here are located in areas that were not colonized by A. adenophora until the 1970s or 1980s (Wang and Wang, 2006). Our results indicate that A. adenophora has two main effects on Keteleeria evelyniana-dominated forests: (1) it reduces the recruitment of K. evelyniana (Fig. 7A and B), and (2) it impoverishes the species diversity in the forest understory (Fig. 7C–E). We found that K. evelyniana forests not only lack young individuals (with a total absence of established seedlings in the age class of 0–5 years and very low values for the age classes of 5–10 and 10–15 years; Fig. 6), but also exhibit a negative correlation between the coverage and density of A. adenophora and the observed number of K. evelyniana seedlings (Fig. 7A and B). These findings suggest either that A. adenophora invaded forests of K. evelyniana in central Yunnan during the most recent 10–15 years, or that this invasion has become very aggressive in the last 10–15 years.
Regeneration of Keteleeria evelyniana forests is limited by several factors (Fig. S3). In K. evelyniana evergreen coniferous forests (Type 1), regeneration is limited by medium to severe disturbances with average invasive species coverages ranging from 55% to 95%. In K. evelyniana–Pinus armandii–C. glaucoides evergreen coniferous and broad-leaved mixed forests (Type 3) and in K. evelyniana–Pinus yunnanensis evergreen coniferous forests (Type 4), the intensity of disturbances varies from medium to severe, with invasive species coverages ranging from 25% to 100% and 40%–95%, respectively, leading to poor seedling recruitment. Although K. evelyniana–Pinus massoniana evergreen coniferous forests (Type 2) lack invasive species and the number of established saplings (ages of 5–10) surpasses those in the other three forest types, regeneration is nonetheless hampered. This is attributed to the native shrub Camellia brevistyla densely populating the understory, shading the light and limiting both space and nutrient availability in the soil.
Disturbances are a key component for the introduction of Ageratina adenophora (Ding et al., 2008). This invasive species, which is stimulated by light to germinate, is vigorous in invading barren or heavily disturbed sites (Wang et al., 1994). In addition, it was found to inhibit native species by altering soil microbial communities (Niu et al., 2007; Wan et al., 2010). Additional factors that enhance A. adenophora invasion include allelopathy (several allelochemicals have been identified in its leachates, Wan et al., 2010), and its high phenotypic plasticity (Zhao et al., 2013). Together, these characteristics of A. adenophora lower the ability to the dominant species (K. evelyniana) to regenerate and reduce species diversity in the forest understory (Fig. 7C–E). The presence of A. adenophora has also changed species composition and abundance in the understory of Pinus yunnanensis forests in Mouding County in central Yunnan Province (Fu et al., 2018).
NRI and NTI indices offer insights into whether a community comprises species that are more or less phylogenetically related than anticipated by chance, aiding researchers in comprehending the processes influencing community assembly and diversity (Webb et al., 2002; Swenson, 2009). Darwin's ‘naturalization hypothesis’ indicated that the lack of competitive exclusion would facilitate the establishment of alien invaders phylogenetically distinct from the native flora (Daehler, 2001). In contrast, his ‘pre-adaptation hypothesis’ posited that nonnative introduced species closely related to native species would be more likely to successfully establish because they might share similar adaptations to the local environment with their native relatives (Darwin, 1859). Darwin's ‘naturalization conundrum’ would be explained by spatial scale (Thuiller et al., 2010; Park et al., 2020). Earlier researchers proposed that closely related species occur in mutually exclusive patterns at small spatial scales (e.g., landscape) where competitive interactions are likely to be more severe (Webb, 2000; Cavender-Bares et al., 2006; Swenson et al., 2007). At larger spatial scales (e.g., continent region), closely related species may be more likely to co-occur due to shared broad environmental preferences and less frequent interspecific competition (Procheş et al., 2008). Recent studies have also shown that the phylogenetic relatedness patterns may also depend on the stage of invasion; for example, in South Africa plant species are more likely to be introduced for cultivation if they are distantly related to the native flora, but the probability of being naturalized was found to be higher for species closely related to the native flora; however, again, the probability of becoming invasive was higher for naturalized species distantly related to the native flora (Omer et al., 2022). In contrast, in China there is a common pattern consistent with the pre-adaptation hypothesis for the introduction–naturalization–invasion continuum (Qian, 2023b). In addition, the possibility that niche conservatism is not always met (e.g. Atwater et al., 2018) adds a further layer of complexity to the expected patterns.
Our analysis of the understory of Keteleeria evelyniana forests in eastern Yunnan indicates that a significantly negative correlation (r = −0.999, p < 0.01) exists between native and invasive species for NRI (Table 2). This suggests that invasive alien species that are closely related to native species in the forest understory might face challenges as invaders, likely due to heightened competition. Thus, our study, which was conducted at a small spatial scale (i.e., plots, habitats), is consistent with Darwin's ‘naturalization hypothesis’ (Park et al., 2020). We have observed that in eastern Yunnan A. adenophora coverage is lower in forest understory with more native species of Asteraceae (D.-M. Du & C.Q. Tang, pers. observ.), which is aligned with our phylogenetic results. Our analysis of phylogenetic relatedness between native and invasive species did not produce statistically significant results for forests in central Yunnan, southcentral Yunnan and southwestern Sichuan. This may be due to severe habitat disturbance, local interactions, and some other ecological factors that would weaken phylogenetic relationships.
4.4. Recommended management measures
This study has shown that human disturbance and Ageratina adenophora invasion threaten the regeneration of Keteleeria evelyniana forests. Although, many populations of K. evelyniana are distributed near roads and villages and, thus, cannot be completely protected, human disturbance and A. adenophora invasion can be limited by establishing local protected areas at fragmented sites. We recommend establishing such local protected areas at four sites: (1) Wenquanlongshan, Anning City, (2) Lvchong scenic site in Fuxianhu, Chengjiang City, (3) Jinning District, Kunming City, (4) Guangnan Village, Qidian Xiang, Chenggong District, Kunming City, in central Yunnan. We also recommend that A. adenophora should be removed from habitats by pulling and digging in the four sites mentioned above. Chemical control of A. adenophora may also prove effective (Yang et al., 2017). Note that previous research has demonstrated biological control of A. adenophora is generally ineffective (e.g., Procecidochares utilis; Yuan et al., 2021b). We also recommend utilizing the results on phylogenetic relatedness (NRI) between native and invasive alien species for predicting (and controlling) invasive plants in the habitats of the Qüjing area, eastern Yunnan. Finally, it is crucial to establish K. evelyniana seedling nurseries for population recovery and conduct experiments aimed at reintroducing them to their natural environment.
5. Potential limitations and sources of bias
One limitation of our study is that field data was limited to January, May and September in the years 2020 and 2021, which may fail to reflect seasonal variations that influence understory herbaceous species diversity. An additional limitation is that the growth trends of trees were derived from a limited number of tree core samplings. Our results on the phylogenetic relatedness between native and invasive alien plant species might also be constrained by our relatively small sample size, limiting the generalizability of the results. Regardless, our work lays the groundwork for future research to address these concerns.
6. Conclusions
The stands of four forest types containing Keteleeria evelyniana as the first dominant are multi-layered, and severely fragmented in their native distribution range. None of the forest communities are in protected areas. K. evelyniana populations lack seedlings/saplings. Trees of K. evelyniana in younger age-classes generally show faster ring growth than older trees at the same age, which might reflect the effects of global climate change. The invasive alien plant Ageratina adenophora prevents K. evelyniana seedling establishment and destabilizes the forests. Invasive alien species that are closely related to native species in the forest understory may be less successful invaders because they compete more intensively in the eastern Yunnan area. We recommend to control this invasive species at the habitats for ensuring seedling/sapling recruitments and conserving the populations and forests of K. evelyniana. The findings will be also practicable in conservation management measures for other vulnerable plants that have similar population dynamics and grow in fragile unprotected forest ecosystems.
CRediT authorship contribution statement
Cindy Q. Tang: Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Formal analysis, Conceptualization. Min-Rui Du: Investigation, Formal analysis, Data curation. Huan-Chong Wang: Writing – review & editing, Supervision, Investigation. You-Cai Shi: Investigation. Jia-Le Zeng: Investigation. Shu-Li Xiao: Investigation. Peng-Bin Han: Investigation. Jian-Ran Wen: Investigation. Shi-Qian Yao: Investigation. Ming-Chun Peng: Investigation. Chong-Yun Wang: Investigation. Yong-Ping Li: Investigation. Jordi López-Pujol: Writing – review & editing, Writing – original draft, Visualization, Validation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was funded by the Major Program for Basic Research Project of Yunnan Province, China, grant number 202101BC070002, and the Special Foundation for National Science and Technology Basic Resources Investigation of China, grant number 2019FY202300.
Footnotes
Peer review under responsibility of Editorial Office of Plant Diversity.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2024.02.006.
Contributor Information
Cindy Q. Tang, Email: cindytang@ynu.edu.cn.
Huan-Chong Wang, Email: hchwang@ynu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- Atwater D.Z., Ervine C., Barney J.N. Climatic niche shifts are common in introduced plants. Nat. Ecol. Evol. 2018;2:34–43. doi: 10.1038/s41559-017-0396-z. [DOI] [PubMed] [Google Scholar]
- Auld B.A. Eupatorium weed species in Australia. PANS (Pest. Artic. News Summ.) 1970;16:82–86. [Google Scholar]
- Averyanov L.V., Nguyen T.H., Sinh K.N., et al. Gymnosperms of Laos. Nord. J. Bot. 2014;32:765–805. doi: 10.1111/njb.00498. [DOI] [Google Scholar]
- Bai F., Chrisholm R., Sang W., et al. Spatial risk assessment of alien invasive plants in China. Environ. Sci. Technol. 2013;47:7624–7632. doi: 10.1021/es400382c. [DOI] [PubMed] [Google Scholar]
- Bai W., Xu H., Wu Q., et al. Ecological study of Keteleeria davidiana populations of Yachang forest farm in Guangxi Zhuang autonomous region. J. Nanjing For. Univ. (Natural Sci. Edition) 2017;41:71–76. [Google Scholar]
- Bellard C., Cassey P., Blackburn T.M. Alien species as a driver of recent extinctions. Biol. Lett. 2016;12 doi: 10.1098/rsbl.2015.0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokhina N.I., Bondarenko O. Fossil plant assemblages from the Pliocene of southern Primory'e Rregion (Russian Far East): implications for reconstruction of plant communities and their environments. Acta Palaeobot. 2011;51:19–37. [Google Scholar]
- Cavender-Bares J., Keen A., Miles B. Phylogenetic structure of Floridian plant communities depends on taxonomic and spatial scale. Ecology. 2006;87:S109–S122. doi: 10.1890/0012-9658(2006)87[109:psofpc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Carrière A.M.B. 2021. Keteleeria Fortunei.http://temperate.theferns.info/plant/Keteleeria+fortunei Available at online. [Google Scholar]
- Chen J., Ma F., Zhang Y., et al. Spatial distribution patterns of invasive alien species in China. Glob. Ecol. Conserv. 2021;26 [Google Scholar]
- Chen X., Wang G.Y., Peng P.H., et al. Effects of taxonomic and phylogenetic diversity of resident Pinus yunnanensis communities on Ageratina adenophora invasion in the Panxi region, Sichuan Province. Biodivers. Sci. 2021;29:865–874. [Google Scholar]
- Chuan H.-Y., Jia D.-R., Pu J., et al. Structural characteristics of Keteleeria davidiana forest communities in Xinping, Yunnan. Chin. J. Plant Ecol. 2021;45:207–212. doi: 10.17521/cjpe.2020.0196. [DOI] [Google Scholar]
- Creech D. 2016. Keteleeria evelyniana.https://dcreechsite.com/2016/12/11/keteleeria-evelyniana/#:∼:text=Keteleer%20(1813%2D1903)%2C,trees%20reaching%2035%20m%20tall Available at online: [Google Scholar]
- Daehler C.C. Darwin's naturalization hypothesis revisited. Am. Nat. 2001;158:324–330. doi: 10.1086/321316. [DOI] [PubMed] [Google Scholar]
- Darji T.B., Adhikari B., Pathak S., et al. Phytotoxic effects of invasive Ageratina adenophora on two native subtropical shrubs in Nepal. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-92791-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. Murray; 1859. On the Origin of Species by Means of Natural Selection. [Google Scholar]
- Ding H., Xu H.-G., Liu Z.-L. Impacts of invasion of Eupatorium adenophorum on vegetation diversity. J. Ecol. Rural Environ. 2007;22(29–35):75. [Google Scholar]
- Ding J., Mack R.N., Lu P., et al. China's booming economy is sparking and accelerating biological invasions. Bioscience. 2008;58:317–324. [Google Scholar]
- Fern K. 2022. Tropical Plants Database.http://tropical.theferns.info/viewtropical.php?id=Keteleeria+evelyniana Available from website. [Google Scholar]
- Fu D., Wu X., Huang N., et al. Effects of the invasive herb Ageratina adenophora on understory plant communities and tree seedling growth in Pinus yunnanensis forests in Yunnan, China. J. For. Res. 2018;23:112–119. doi: 10.1080/13416979.2018.1429202. [DOI] [Google Scholar]
- Fu L. In: Flora of China. Wu Z., Peter H.R., editors. Missouri Botanical Garden Press, St. Louis, and Science Press; Beijing: 1999. Keteleeria; pp. 42–44. [Google Scholar]
- Gazol A., Camarero J.J., Gutiérrez E., et al. Distinct effects of climate warming on populations of silver fir (Abies alba) across Europe. J. Biogeogr. 2015;42:1150–1162. [Google Scholar]
- González-Moreno P., Pino J., Gassó N., et al. Landscape context modulates alien plant invasion in Mediterranean forest edges. Biol. Invasions. 2013;15:547–557. doi: 10.1007/s10530-012-0306-x. [DOI] [Google Scholar]
- Gosper H. NSW Agriculture; 2003. Crofton Weed.http://www.agric.nsw.gov.au/reader/weed-list [Google Scholar]
- Hao Q., Ma J.-S. Invasive alien plants in China: an update. Plant Divers. 2023;45:117–121. doi: 10.1016/j.pld.2022.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Hu D., Liu X., et al. Studies on distribution pattern of Keteleeria davidiana in Zhangheyuan. J. Green Sci. Techn. 2018;13:13–15. [Google Scholar]
- Hiep N.T., Loc P.K., Luu N.D.T., et al. Fauna & Flora International, Vietnam Programme; Hanoi: 2004. Vietnam Conifers: Conservation Status Review. [Google Scholar]
- Hu G.X., Xiang C.L., Liu E.D. Invasion status and risk assessment for Salvia tiliifolia, a recently recognised introduction to China. Weed Res. 2013;53:355–361. [Google Scholar]
- Huzita K., Kasama T. Geological Survey of Japan; 1983. Geology of Kobe District. Quadrangle Series; p. 115. [Google Scholar]
- Jin Y., Qian H. V.PhyloMaker: an R package that can generate very large phylogenies for vascular plants. Ecography. 2019;42:1353–1359. doi: 10.1111/ecog.04434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Qian H. V.PhyloMaker2: an updated and enlarged R package that can generate very large phylogenies for vascular plants. Plant Divers. 2022;44:335–339. doi: 10.1016/j.pld.2022.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kembel S.W., Cowan P.D., Helmus M.R., et al. Picante: R tools for integrating phylogenies and ecology. picante: R tools for integrating phylogenies and ecology. Bioinformatics. 2010;26:1463–1464. doi: 10.1093/bioinformatics/btq166. [DOI] [PubMed] [Google Scholar]
- Keyimu M., Wei J., Zhang Y., et al. Climate signal shift under the influence of prevailing climate warming – evidence from Quercus liaotungensis on Dongling Mountain, Beijing, China. Dendrochronologia. 2020;60 [Google Scholar]
- Klippel L., Krusic P.J., Brandes R., et al. High elevation inter-site differences in Mount Smolikas tree-ring width data. Dendrochronologia. 2017;44:164–173. doi: 10.1016/j.dendro.2017.05.006. [DOI] [Google Scholar]
- Lande R. Statistics and partitioning of species diversity, and similarity among multiple communities. Oikos. 1996;76:5–13. doi: 10.2307/3545743. [DOI] [Google Scholar]
- Langmaier M., Lapin K. A systematic review of the impact of invasive alien plants on forest regeneration in European temperate forests. Front. Plant Sci. 2020;11 doi: 10.3389/fpls.2020.524969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.-L. Epoch Publishing; Taipei: 1975. Flora of Taiwan, Vol. 1, Parts 1–8. [Google Scholar]
- Li X.-S., Zhao A.-N., Dang C.-L., Peng M.-C. A study on the structure and regeneration of Keteleeria evelyniana mixed forest in Xishan, Kunming. J. Yunnan Univ. (Nat. Sci.) 2013;35(4):549–557. [Google Scholar]
- Lin Q., Xiao C., Ma J.-S. Science Data Bank; 2022. A Dataset on Catalogue of Alien Plants in China [DS/OL]http://cstr.cn/31253.11.sciencedb.01711.CSTR:31253.11.sciencedb.01711 2022. [Google Scholar]
- Liu J., Yuan L., Shi Q., et al. Growth rule of Keteleeria evelyniana planted forest in Xishan of Kunming. Inner Mongolia. For. Investig. Design. 2016;39:31–35. doi: 10.13387/j.cnki.nmld.2016.06.011. [DOI] [Google Scholar]
- Liu Y.S., Guo S.X., Ferguson D.K. A catalogue of Cenozoic megafossil plants in China. Palaeontogr. Abt. B Palaeophytol. 1996;238:141–179. [Google Scholar]
- Loc P.K., The P.V., Long P.K., et al. Native conifers of Vietnam – a review. Pakistan J. Bot. 2017;49:2037–2068. [Google Scholar]
- Luscombe D., Yang Y. Keteleeria davidiana var. formosana. Available from the website: “Threatened Conifers of the World”. 2019. https://threatenedconifers.rbge.org.uk/conifers/keteleeria-davidiana-var.-formosana
- Luu N.D.T., Thomas P.I. 2004. Conifers of Vietnam. Darwin Initiative. ISBN 1-872291-64-3.http://www.ceh.ac.uk/sections/bm/conifer_manual.html Available from website: [Google Scholar]
- Martin P.H., Canham C.D., Marks P.L. Why forests appear resistant to exotic plant invasions: intentional introductions, stand dynamics, and the role of shade tolerance. Front. Ecol. Environ. 2009;7:142–149. doi: 10.1890/070096. [DOI] [Google Scholar]
- Mathewes R.W., Greenwood D.R., Archibald S.B. Paleoenvironment of the Quilchena flora, British Columbia, during the early Eocene climatic optimum. Can. J. Earth Sci. 2016;53:1–17. [Google Scholar]
- McCune B., Mefford M.J. Wild Blueberry Media; Corvallis, Oregon, U.S.A: 2016. PC-ORD: Multivariate Analysis of Ecological Data, Version 7.0 for Windows. [Google Scholar]
- McMahon S.M., Parker G.G., Miller D.R. Evidence for a recent increase in forest growth. Proc. Natl. Acad. Sci. U.S.A. 2010;107:3611–3615. doi: 10.1073/pnas.0912376107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H.W., Manchester S.R. Vol. 141. Univ. California Publications Geol. Sci.; 1997. pp. 1–364. (The Oligocene Bridge Creek Flora of the John Day Formation, Oregon). [Google Scholar]
- Nguyen T., Tran D., Nguyen T., et al. Natural Science and Technology Publishing House; Hanoi: 2007. Vietnam Red Data Book–Part II. Plants. [Google Scholar]
- Ning Q.-R., Gong X.-W., Li M.-Y., et al. Differences in growth pattern and response to climate warming between Larix olgensis and Pinus koraiensis in Northeast China are related to their distinctions in xylem hydraulics. Agric. For. Meteorol. 2022;312 [Google Scholar]
- Niu H.B., Liu W.X., Wan F.H., et al. An invasive aster (Ageratina adenophora) invades and dominates forest understories in China: altered soil microbial communities facilitate the invader and inhibit natives. Plant Soil. 2007;294:73–85. doi: 10.1007/s11104-007-9230-8. [DOI] [Google Scholar]
- Omer A., Fristoe T., Yang Q., et al. The role of phylogenetic relatedness on alien plant success depends on the stage of invasion. Nat. Plants. 2022;8:906–914. doi: 10.1038/s41477-022-01216-9. [DOI] [PubMed] [Google Scholar]
- Ozaki K. On the paleoenvironments of the late Miocene tatsumitoge flora. Sci. Rep. Yokohama Nat. Univ. Sec. 1981;2:47–75. [Google Scholar]
- Park D.S., Feng X., Maitner B.S., et al. Darwin's naturalization conundrum can be explained by spatial scale. Proc. Natl. Acad. Sci. U.S.A. 2020;117:10904–10910. doi: 10.1073/pnas.1918100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D.S., Potter D. A test of Darwin's naturalization hypothesis in the thistle tribe shows that close relatives make bad neighbors. Proc. Natl. Acad. Sci. U.S.A. 2013;110:17915–17920. doi: 10.1073/pnas.1309948110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielou E.C. Wiley; New York: 1969. An Introduction to Mathematical Ecology. [Google Scholar]
- Poudel A.S., Jha P.K., Shrestha B.B., et al. Biology and management of the invasive weed Ageratina adenophora (Asteraceae): current state of knowledge and future research needs. Weed Res. 2019;59:79–92. [Google Scholar]
- Procheş S., Wilson J.R.U., Richardson D.M., et al. Searching for phylogenetic pattern in biological invasions. Global Ecol. Biogeogr. 2008;17:5–10. [Google Scholar]
- Qin H., Yang Y., Dong S., et al. Threatened species list of China's higher plants. Biodivers. Sci. 2017;25:696–744. doi: 10.17520/biods.2017144. [DOI] [Google Scholar]
- Qian H. Global patterns of phylogenetic relatedness of invasive flowering plants. Divers. Distrib. 2023;29:1106–1117. [Google Scholar]
- Qian H. Patterns of phylogenetic relatedness of non-native plants across the introduction–naturalization–invasion continuum in China. Plant Divers. 2023;45:169–176. doi: 10.1016/j.pld.2022.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2021. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ [Google Scholar]
- Raghubanshi A.S., Tripathi A. Effect of disturbance, habitat fragmentation and alien invasive plants on floral diversity in dry tropical forests of vindhyan highland: a Review. Trop. Ecol. 2009;50:57. doi: 10.1155/2011/297097. [DOI] [Google Scholar]
- Ran J.-H., Shen T.-T., Wu H., et al. Phylogeny and evolutionary history of Pinaceae updated by transcriptomic analysis. Mol. Phylogenet. Evol. 2018;129:106–116. doi: 10.1016/j.ympev.2018.08.011. [DOI] [PubMed] [Google Scholar]
- Rember B. Vol. 32. A Publication Idaho Native Plant Soc.; 2010. pp. 1–3. (Lake Clarkia Fossil Sites). [Google Scholar]
- Rogling A., Brühlhart H., Bräker O.U., et al. Effects of irrigation on diameter growth and vertical resin duct production in Pinus sylvestris L. on dry sites in the central Alps. For. Ecol. Manag. 2003;175:285–296. Switzerland. [Google Scholar]
- Rollinson C.R., Kaye M.W., Canham C.D. Interspecific variation in growth responses to climate and competition of five eastern tree species. Ecology. 2016;97:1003–1011. [PubMed] [Google Scholar]
- Rubino D.L., McCarthy B.C. Dendroclimatological analysis of white oak (Quercus alba L., Fagaceae) from an old-growth forest of southeastern Ohio. U.S.A. J. Torrey Bot. Soc. 2000;127:240–250. doi: 10.2307/3088761. [DOI] [Google Scholar]
- Rusterholz H.-P., Schneuwly J., Baur B. Invasion of the alien shrub Prunus laurocerasus in suburban deciduous forests: effects on native vegetation and soil properties. Acta Oecol. 2018;92:44–51. doi: 10.1016/j.actao.2018.08.004. [DOI] [Google Scholar]
- Salzer M.W., Hughes M.K., Bunn A.G., et al. Recent unprecedented tree-ring growth in bristlecone pine at the highest elevations and possible causes. Proc. Natl. Acad. Sci. U.S.A. 2009;106:20348–20353. doi: 10.1073/pnas.0903029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang W., Zhu L., Axmacher J.C. Invasion pattern of Eupatorium adenophorum Spreng in southern China. Biol. Invasions. 2010;12:1721–1730. doi: 10.1007/s10530-009-9584-3. [DOI] [Google Scholar]
- Sarris D., Christodoulakis D., Körner C. Recent decline in precipitation and tree growth in the eastern Mediterranean. Global Change Biol. 2007;13:1187–1200. [Google Scholar]
- Smith S.A., Brown J.W. Constructing a broadly inclusive seed plant phylogeny. Am. J. Bot. 2018;105:302–314. doi: 10.1002/ajb2.1019. [DOI] [PubMed] [Google Scholar]
- Strauss S.Y., Webb C.O., Salamin N. Exotic taxa less related to native species are more invasive. Proc. Natl. Acad. Sci. U.S.A. 2006;103:5841–5845. doi: 10.1073/pnas.0508073103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson N.G. Phylogenetic resolution and quantifying the phylogenetic diversity and dispersion of communities. PLoS One. 2009;4 doi: 10.1371/journal.pone.0004390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson N.G., Enquist B.J., Thompson J., et al. The influence of spatial and size scale on phylogenetic relatedness in tropical forest communities. Ecology. 2007;88:1770–1780. doi: 10.1890/06-1499.1. [DOI] [PubMed] [Google Scholar]
- Tang C.Q. Vol. 11. Springer; Dordrecht: 2015. The subtropical vegetation of southwestern China: plant distribution, diversity and ecology. (Plants and Vegetation). [DOI] [Google Scholar]
- Tang C.Q., Lu X., Du M.-R., et al. Forest characteristics and population structure of a threatened palm tree Caryota obtusa in the karst forest ecosystem of Yunnan, China. J. Plant Ecol. 2022;15:829–843. doi: 10.1093/jpe/rtab117. [DOI] [Google Scholar]
- Tang C.Q., Matsui T., Ohashi H., et al. Identifying long-term stable refugia for relict plant species in East Asia. Nat. Commun. 2018;9:4488. doi: 10.1038/s41467-018-06837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C.Q., He L.-Y., Gao Z., et al. Habitat fragmentation, degradation, and population status of endangered Michelia coriacea in Southeastern Yunnan, China. Mt. Res. Dev. 2011;31:343–350. [Google Scholar]
- Terral J.-F., Arnold-Simard G. Beginnings of olive cultivation in eastern Spain in relation to Holocene bioclimatic changes. Quat. Res. 1996;46:176–185. [Google Scholar]
- Terral J.-F., Durand A. Bio-archaeological evidence of olive tree (Olea europaea L.) irrigation during the middle ages in southern France and north eastern Spain. J. Archaeol. Sci. 2006;33:718–724. [Google Scholar]
- Thabeet A., Vennetier M., Gadbin-Henry C., et al. Response of Pinus sylvestris L. to recent climatic events in the French Mediterranean region. Trees (Berl.) 2009;23:843–853. [Google Scholar]
- Thomas C.D., Cameron A., Green R.E., et al. Extinction risk from climate change. Nature. 2004;427:145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- Thomas P. 2013. Keteleeria evelyniana. The IUCN Red List of Threatened Species 2013: e.T42307A2971138. 10.2305/IUCN.UK.2013-1.RLTS.T42307A2971138.en. [Google Scholar]
- Thomas P. Keteleeria evelyniana, from the website: “Threatened conifers of the world”. 2019. http://threatentedconifers.rbge.org.uk/conifers/keteleeria-evelyniana
- Thuiller W., Gallien L., Boulangeat I., et al. Resolving Darwin's naturalization conundrum: a quest for evidence. Divers. Distrib. 2010;16:461–475. [Google Scholar]
- Wan F.H., Liu W.X., Guo J.Y., et al. Invasive mechanism and control strategy of Ageratina adenophora (Sprengel) Sci. China Life Sci. 2010;53:1291–1298. doi: 10.1007/s11427-010-4080-7. [DOI] [PubMed] [Google Scholar]
- Wang H.J., He P., Ma J.L. An investigation and research report on the dissemination of Ageratina adenophora on rangeland areas in Liangshan District of Sichuan Province. Grassland China. 1994;1:62–64. [Google Scholar]
- Wang J.J. In: Biology and Management of Invasive Alien Species in Agriculture and Forestry. Wan F.H., Zhang X.B., Guo J.Y., editors. Science Press; Beijing: 2005. Crofton weed – Eupatorium adenophorum spreng; pp. 650–661. [Google Scholar]
- Wang R. PhD Dissertation. Institute of Botany, Chinese Academy of Sciences; Beijing: 2006. Historical reconstruction of invasion and expansion and potential spread of some threatening invasive alien species in China. [Google Scholar]
- Wang R., Wang Y.Z. Invasion dynamics and potential spread of the invasive alien plant species Ageratina adenophora (Asteraceae) in China. Divers. Distrib. 2006;12:397–408. [Google Scholar]
- Webb C.O. Exploring the phylogenetic structure of ecological communities: an example for rain forest trees. Am. Nat. 2000;156:145–155. doi: 10.1086/303378. [DOI] [PubMed] [Google Scholar]
- Webb C.O., Ackerly D.D., McPeek M.A., et al. Phylogenies and community ecology. Annu. Rev. Ecol. Syst. 2002;33:475–505. doi: 10.1146/annurev.ecolsys.33.010802.150448. [DOI] [Google Scholar]
- Wei Q.-S., Wu M., Huang Y.-C., et al. Growth regularity of Keteleeria davidiana natural forest. J. Northwest For. Univ. 2014;30:140–146. doi: 10.3969/j.issn.1001-7461.2014.05.27. [DOI] [Google Scholar]
- WFO Plant List [WWW Document] https://wfoplantlist.org/taxon/
- Wolff M.A. Kangaroo Press; Kenthurst, New South Wales: 1999. Winning the War of Weeds: the Essential Gardener's Guide to Weed Identification and Control. [Google Scholar]
- Xie Y., Li Z.Y., William P.G., et al. Invasive species in China-an overview. Biodivers. Conserv. 2001;10:1317–1341. [Google Scholar]
- Xu H., Qiang S., Genovesi P., et al. An inventory of invasive alien species in China. NeoBiota. 2012;15:1–26. doi: 10.3897/neobiota.15.3575. [DOI] [Google Scholar]
- Yan X., Liu Q., Shao H., et al. The categorization and analysis on the geographic distribution patterns of Chinese alien invasive plants. Biodivers. Sci. 2014;22:667–676. doi: 10.3724/SP.J.1003.2014.14069. [DOI] [Google Scholar]
- Yang G., Gui F., Liu W., et al. In: Biological Invasions and its Management in China (Invading Nature - Springer Series in Invasion Ecology 13) Wan F., Jiang M., Zhan A., editors. Springer Nature; Singapore: 2017. Crofton weed Ageratina adenophora (sprengel) pp. 111–129. [Google Scholar]
- Yang Q.-E. In: Wu Z., Peter H.R., editors. Vol. 4. Missouri Botanical Garden Press, St. Louis, and Science Press; Beijing: 1999. Keteleeria evelyniana; pp. 42–44. (Flora of China). [Google Scholar]
- Yang Y. An updated red list assessment of gymnosperms from China (Version 2021) Biodivers. Sci. 2021;29:1599–1606. [Google Scholar]
- Yesemuratova R.X. Rare and endemic plants of the relic mountain of Sultan-Uvays. Am. J. Plant Sci. 2021;12:1–6. doi: 10.4236/ajps.2021.121001. [DOI] [Google Scholar]
- Yessoufou K., Gere J., Daru B.H., van der Bank M. Differences in evolutionary history translate into differences in invasion success of alien mammals in South Africa. Ecol. Evol. 2014;4:2115–2123. doi: 10.1002/ece3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C., Wang Q., Chen Y., et al. Impacts of a biocontrol agent on invasive Ageratina adenophora in Southwest China: friend or foe? Biol. Control. 2021;152 [Google Scholar]
- Yuan D., Zhu L., Cherubini P., et al. Species-specific indication of 13 tree species growth on climate warming in temperate forest community of northeast China. Ecol. Indicat. 2021;133 [Google Scholar]
- Zahner R., Lotan J.E., Baughman W.D. Earlywood-latewood features of red pine grown under simulated drought and irrigation. For. Sci. 1964;10:361–370. doi: 10.1093/forestscience/10.3.361. [DOI] [Google Scholar]
- Zhang A., Hu X., Yao S., et al. Alien, naturalized and invasive plants in China. Plants. 2021;10:2241. doi: 10.3390/plants10112241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Li D., Xia S., et al. Biological trait of alien invasive plants in Yunnan Province. Guihaia. 2018;38:269–280. doi: 10.11931/guihaia.gxzw201703038. [DOI] [Google Scholar]
- Zhang X.-H., Xu F., Yang Y.M., et al. Analysis and evaluation of nutritional components and total flavonoids in burgeon of Keteleeria evelyniana. Mast. Food Res. Devel. 2014;35:109–111. doi: 10.3969/j.issn.1005-6521.2014.20.028. [DOI] [Google Scholar]
- Zhao L. Central China Normal University; 2009. Preliminary Ecology Study on Keteleeria davidiana Community in Hubei Province. Master Thesis. [Google Scholar]
- Zhao X., Liu W., Zhou M. Lack of local adaptation of invasive crofton weed (Ageratina adenophora) in different climatic areas of Yunnan Province, China. J. Plant Ecol. 2013;6:316–322. [Google Scholar]
- Zhou Z.-Y., Liu W.-X., Pei G., et al. Phenolics from Ageratina adenophora roots and their phytotoxic effects on Arabidopsis thaliana seed germination and seedling growth. J. Agric. Food Chem. 2013;61:11792–11799. doi: 10.1021/jf400876j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.