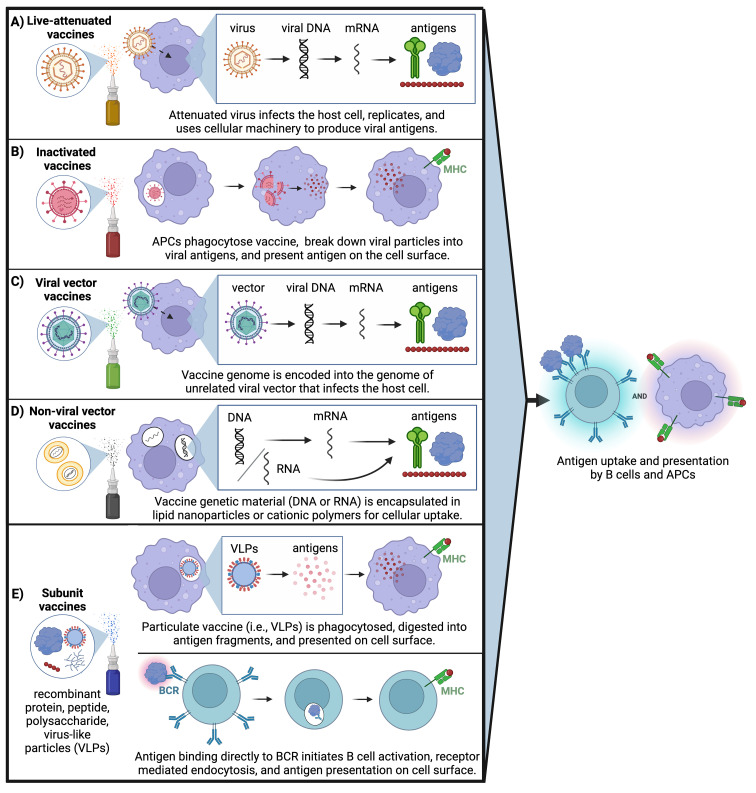
Figure 4.
Mucosal vaccine approaches: All mucosal vaccines that are clinically approved are based on live attenuated or inactivated pathogens; however, ongoing clinical trials are investigating other strategies to overcome challenges associated with pathogenic vaccines. (A) Live attenuated virus infects the host cell, undergoes replication, and uses cellular machinery to produce viral antigens. (B) Inactivated virus is phagocytosed by APCs, broken down into viral antigens, then presented on the cell surface. (C) Vaccine genome is encapsulated into an unrelated viral vector that infects the host cell then uses cellular machinery to produce viral antigens. (D) Genetic content from the vaccine (either DNA or RNA) is encapsulated in lipid nanoparticles or cationic polymers for cellular uptake followed by translation of viral proteins. (E) Subunit vaccines can utilize two delivery mechanisms: Particulate vaccine containing virus-like particles is phagocytosed by APCs and digested into viral antigen fragments followed by antigen presentation on the cell surface. Or, recombinant and/or particulate subunit vaccines can directly bind to the B cell receptor (BCR) on the surface of B cells followed by receptor-mediated endocytosis and antigen presentation on the cell surface. (Created with BioRender.com).