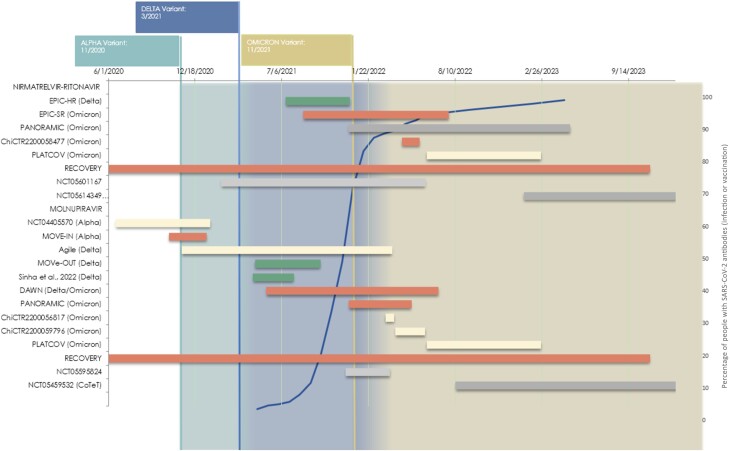
Figure 1.
Timeline of trials testing nirmatrelvir/ritonavir and/or molnupiravir in patients with coronavirus disease 2019. Red indicates negative results for hospitalization and death, green indicates positive results for hospitalization and death, yellow indicates softer outcomes (eg, viral clearance), and gray indicates no published results. The width of the bar represents the time between the start and end of enrollment. The blue line in the background indicates the percentage of the population with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies (https://COVID.cdc.gov/COVID-data-tracker/#nationwide-blood-donor-seroprevalence). Abbreviations: RECOVERY, randomised evaluation of covid-19 therapy; EPIC-SR, evaluation of protease inhibition for covid-19 in standard-risk patients; EPIC-HR, evaluation of protease inhibition for covid-19 in high-risk patients.