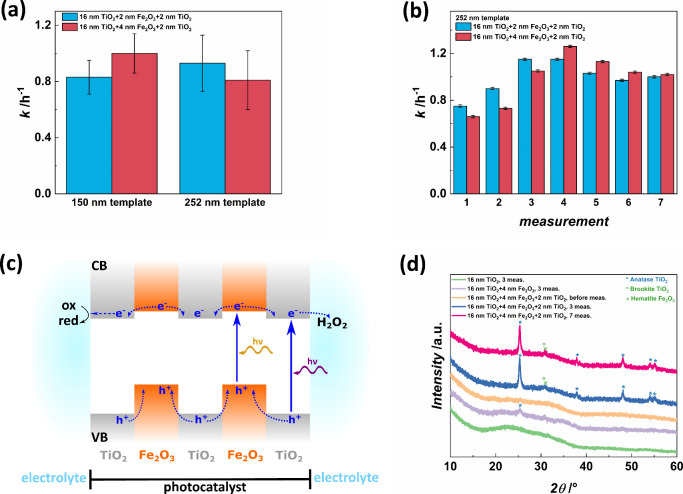
Figure 5.
(a) The mean photocatalytic activity of TiO2–Fe2O3–TiO2 multilayer IOs after three measurements depends on the composition and template size but shows a significant standard deviation. (b) The individual activities during seven consecutive measurements of 16 nm TiO2–2 nm Fe2O3–2 nm TiO2 and 16 nm TiO2–4 nm Fe2O3–2 nm TiO2 multilayer IOs for the 252 nm template increase during the first four measurements, slightly decrease in the following two measurements and are stable afterward. (c) The band structure of TiO2–Fe2O3–TiO2 multilayer IOs depicts trapping of photogenerated holes inside the Fe2O3 layers due to adding another TiO2 layer. Electrons in the CB move toward the TiO2 layers, and those located in the outer layers can induce reductive reactions in the surrounding electrolyte or get scavenged by H2O2 molecules. (d) XRD patterns show anatase TiO2 peaks for Fe2O3 functionalized TiO2 IOs after photocatalysis measurements. Multilayer structures exhibit significantly higher peak intensities, indicating that this composition provokes photoinduced crystallization of the TiO2 layers.