Abstract
Background
Preventing preterm labour is the most important step in preventing preterm birth. Prostaglandins play an important role in labour and birth. Prostaglandin production can be obstructed by inhibition of the cyclo‐oxygenase (COX) enzyme and this may arrest uterine contraction. A Cochrane review on COX inhibitors for the treatment of preterm labour found insufficient data to draw conclusions about its effectiveness.
Objectives
To assess the effectiveness and safety of COX inhibitors for preventing preterm labour in high‐risk women.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trial Register (30 June 2012).
Selection criteria
All published and unpublished randomised trials evaluating administration of any COX inhibitor for prevention of preterm labour in pregnant women at gestational age less than 36 weeks at risk of, but not experiencing, preterm labour. Cluster‐randomised trials were eligible for inclusion. Quasi‐randomised trials and studies with cross‐over designs were excluded.
Data collection and analysis
Two review authors (T Khanprakob and U Sangkomkamhang) independently assessed all potential studies for inclusion. Disagreement was resolved by discussion and, where necessary, by consultation with a third review author. Two review authors independently assessed trial quality and extracted data. Data were checked for accuracy.
Main results
We included one randomised trial (involving 98 women) that evaluated the effectiveness of one type of COX inhibitor (rofecoxib) for preventing preterm birth. The included study did not report any data for one of our primary outcomes: preterm labour. Rofecoxib use was associated with an increased risk for preterm birth and preterm premature rupture of membranes (PPROM). Rofecoxib was associated with a higher risk of oligohydramnios and low fetal urine production but the effects were reversible after stopping treatment. There were no differences in the number of women who discontinued treatment before 32 weeks of gestation. There was no difference in neonatal morbidities and admission to neonatal intensive care unit. There were no maternal adverse effects or perinatal mortalities in either group.
Authors' conclusions
There was very little evidence about using COX inhibitors for preventing preterm labour. There are inadequate data to make any recommendation about using COX inhibitor in practice to prevent preterm labour. Future research should include follow‐up of the babies to examine the short‐term and long‐term effects of COX inhibitors.
Keywords: Adult; Female; Humans; Pregnancy; Cyclooxygenase 2 Inhibitors; Cyclooxygenase 2 Inhibitors/therapeutic use; Lactones; Lactones/therapeutic use; Obstetric Labor, Premature; Obstetric Labor, Premature/prevention & control; Randomized Controlled Trials as Topic; Sulfones; Sulfones/therapeutic use
Plain language summary
Cyclo‐oxygenase (COX) inhibitors for preventing labour before full‐term pregnancy
Labour before full term in pregnancy can lead to preterm birth of the baby. Preterm labour describes frequent uterine contractions (at least four in 20 minutes or eight in 60 minutes) and progressive changes in the cervix. If preterm labour is not managed properly, active labour can occur and result in preterm birth, before 37 completed weeks' gestation. Preterm birth is the leading cause of low birthweight, illness and death for newborn babies. Substances called prostaglandins play an important role in the contraction of the muscle of the womb and are important during labour and birth. They are produced by cyclo‐oxygenase (COX), which is an enzyme that increases the level of prostaglandins. Giving COX inhibitors to pregnant women at risk of preterm labour might stop contraction of the womb and allow them to reach full term. We included one small randomised trial (involving 98 women) that involved the drug rofecoxib, which is one type of COX inhibitor. The included study did not report any information about prevention of labour before full‐term pregnancy. However, use of this COX inhibitor was associated with an increased risk of the baby being born before full term. We found insufficient data to make any recommendation about using COX inhibitors for preventing preterm labour. Future research should include the follow‐up of babies to examine the short‐ and longer‐term effects associated with using COX inhibitors during pregnancy.
Background
Description of the condition
Preterm birth is defined as delivery before 37 completed weeks' gestation (Cunningham 2005). Worldwide incidence of preterm birth is about 10% (Beck 2010).
The definition of preterm labour depends on the setting but it generally comprises the following three criteria: uterine contraction frequency of four in 20 minutes or eight in 60 minutes plus progressive change in the cervix, cervical dilatation greater than 1 cm and cervical effacement of 80% or greater (Cunningham 2005).
If preterm labour is not managed properly, active labour can occur and result in preterm birth. Preterm birth is one of the major complications in obstetrics. The mortality rate of preterm infants has continued to decline since the 1960s, but infant mortality associated with preterm birth or low birthweight has not declined (Arias 2003). Preterm birth is also the leading cause of perinatal morbidities such as low birthweight, respiratory distress syndrome, intraventricular haemorrhage, anaemia, etc. Moreover, these complications may cause both major and minor consequences for these newborn infants (Kliegman 2007).
Factors associated with preterm birth include: medical and obstetric complications (Meis 1995), multiple pregnancy (Blondel 2006; Kurdi 2004; Murphy 2007), threatened abortion (Weiss 2004), cigarette smoking (Tsai 2008), short stature, low pre‐pregnancy body mass index (BMI), inadequate weight gain (Kramer 1995; Schieve 2000), young or advanced maternal age (Morken 2005), prolonged walking or standing, strenuous working conditions, long weekly work hours (Luke 1995), stress (Hobel 2003), genetic factors (Hoffman 1999), chorioamnionitis (Ustun 2001), lower genital tract infections (Kiss 2004), history of previous abortion, socioeconomic status and nulliparity (Meis 1995).
Description of the intervention
Prevention of preterm labour is the most important step for preventing preterm birth. Prostaglandins have been proposed as playing an important role in parturition (Gibb 2002; Olson 1995). Prostaglandins have a diverse effect on the uterus (Narumiya 1999). They are commonly considered as uterotonic (increasing myometrial tone) (Olson 2007) but can sometimes act as smooth muscle relaxants (Toppozada 1975).
Cyclo‐oxygenase (COX) is an enzyme in the pathway of prostaglandins synthesis. This pathway can be obstructed by COX inhibitors resulting in the hindrance of prostaglandin production.
How the intervention might work
Prostaglandins are produced from plasma arachidonic acid, which is usually released by the action of the enzymes phospholipases A2 or C. Arachidonic acid can act as substrate for cyclo‐oxygenase‐1 and ‐2 (COX‐1 and ‐2) (Smith 1996). These enzymes are the target of many non‐steroidal anti‐inflammatory drugs (NSAIDs) (Vane 1998).
The effect of prostaglandins on tissue targets is influenced by prostaglandin receptors. The prostaglandin family of receptors is classified according to the specificity of binding of a given receptor to a particular prostaglandin. These receptors are thromboxane A2(TP), PGD2(DP), prostacyclin or PGI2(IP), PGF2alpha(FP), and EP1 to EP4 (PGE2) (Narumiya 1999). PGE2 and PGI2 have been shown to cause relaxation of vascular smooth muscle and vasodilatation in many circumstances (Henry 2005; Williams 1994). Thus, either the generation of specific prostaglandins or the relative expression of the various prostaglandin receptors may determine the responses of human myometrium to prostaglandins (Myatt 2004). In addition to changes with gestation, several studies have shown that there may be regional changes in the upper and lower uterine segments (Brodt‐Eppley 1999; Grigsby 2006). Thus, it is entirely possible that prostanoids contribute to myometrial relaxation at one stage of pregnancy and to regional myometrial contraction of the fundus after the initiation of parturition. Many investigators have accepted and fostered the view that prostaglandins, particularly PGF2alpha and PGE2, are involved in the process of labour. The following evidence may support the role of COX inhibitors in preventing preterm labour.
Levels of prostaglandins in amniotic fluid, maternal plasma and the uterus increase during labour (Gibb 1998).
Prostaglandins cause abortion or labour (Novy 1980).
An observational study by Loudon found that COX‐2 inhibitors were effective in treating preterm labour (Loudon 2003).
Prostaglandin treatment of myometrial smooth muscle tissues in vitro sometimes causes contraction, dependent on the prostanoid tested and the physiological status of the tissue treated (Myatt 2004).
However, like oxytocin, prostaglandins produced directly in or adjacent to myometrial tissue are likely to play a major role in the effectiveness of myometrial contractions of active labour once labour is initiated. Based on the evidence that during the parturition process prostaglandins play an important role in labour, COX inhibitors may help to relieve myometrial tone (tocolytic effect) and result in cessation of labour (Dawood 1993; Olson 2003). COX‐2 progressively increases expression while gestational age increases, so there is a potential role for COX‐2 in normal parturition (Vermillion 2005). Thus, selective COX‐2 inhibitors may be more effective in preventing uterine contraction than non‐selective COX inhibitors.
There are potential risks reported when using COX inhibitors in pregnancy owing to COX inhibitors crossing the placenta to influence prostaglandins synthesis in the fetus. One systematic review (Loe 2005) reported no significances difference in adverse fetal effects when using COX inhibitors (indomethacin) as a tocolytic drug (intraventricular haemorrhage, patent ductus arteriosus, necrotising enterocolitis or neonatal mortality). One Cochrane review (King 2005) found insufficient data to address the adverse effects of maternally used COX inhibitors on fetuses and newborns. The potential adverse effects appeared to increase in incidence when a COX inhibitor was used for longer than 72 hours and after 30 weeks of gestation (Vermillion 1997).
Why it is important to do this review
There is no Cochrane review addressing the effectiveness and safety of COX inhibitors in preventing preterm labour. Another Cochrane review on the treatment of preterm labour using COX inhibitors found insufficient data to draw conclusions about effectiveness and limited information on serious maternal outcomes such as death, cardiac arrest, respiratory arrest and admission to intensive care unit (King 2005). There is no Cochrane review on the effectiveness and safety of antenatal COX inhibitors for preventing preterm labour in high‐risk women.
Objectives
To assess the effectiveness and safety of COX inhibitors for preventing preterm labour in high‐risk women.
Methods
Criteria for considering studies for this review
Types of studies
Randomised trials, including cluster‐randomised trials, evaluating administration of any COX inhibitors for preventing preterm labour. We excluded quasi‐randomised trials and studies with cross‐over designs.
Types of participants
Pregnant women at gestational age less than 36 weeks at risk of, but not experiencing, preterm labour.
Types of interventions
Administration of COX inhibitors compared with placebo or any other interventions (including conservative management) for preventing preterm labour.
Types of outcome measures
Primary outcomes
Preterm labour (less than 37 completed weeks).
Preterm birth (less than 37 completed weeks).
Secondary outcomes
Maternal outcomes
Preterm labour at gestational age of 34 to less than 37 completed weeks.
Preterm labour at gestational age of 28 to less than 34 completed weeks.
Preterm labour at gestational age of 22 to less than 28 completed weeks.
Labour‐free interval.
Preterm birth less than 34 weeks.
Adverse effects.
Length of hospital stay.
Pregnant women's satisfaction.
Neonatal outcomes
-
Birthweight:
Low birthweight: birthweight less than 2500 grams.
Very low birthweight: birthweight less than 1500 grams.
Extremely low birthweight: birthweight less than 1000 grams.
Neonatal morbidity (the presence of one or more of the following conditions: premature closure of ductus arteriosus, patent ductus arteriosus, sepsis, necrotising enterocolitis, intraventricular haemorrhage, respiratory distress syndrome).
Admission to neonatal intensive care unit.
Perinatal mortality.
Long‐term outcomes, for example developmental delay, cerebral palsy, educational attainment, etc.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register by contacting the Trials Search Co‐ordinator (20 January 2012).
The Cochrane Pregnancy and Childbirth Group's Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the 'Specialized Register' section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two review authors (Thirawut Khanprakob (TK) and Ussanee Sangkomkamhang (US)) independently assessed all potential studies identified as a result of the search strategy for inclusion. We resolved any disagreement through discussion or consultation with a third review author.
Data extraction and management
We modified the Cochrane PCG template for data extraction. TK and US extracted the data using the agreed form. We resolved discrepancies through discussion. We used the Review Manager software (RevMan 2011) to double enter all the data.
We attempted to contact authors of the original reports to provide further details when information regarding any of the above is unclear.
Assessment of risk of bias in included studies
TK and US independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assess whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3) Blinding of participants, personnel and outcome assessment (checking for possible performance bias or detection bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered studies to be at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel;
low, high or unclear risk of bias for outcome assessors;
partial for some situations (e.g. data from an unblinded participant been recorded by blinded personnel).
(4) Incomplete outcome data (checking for possible attrition bias owing to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or was supplied by the trial authors, we re‐include missing data in the analyses that we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; 'as treated' analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
We discussed whether missing data greater than 20% might (a) be reasonably expected (acknowledging that with long‐term follow‐up, complete data are difficult to attain), and (b) impact on outcomes.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review were reported);
high risk of bias (where not all the study's pre‐specified outcomes were reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so cannot be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias owing to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
Measures of treatment effect
We carried out statistical analysis using Review Manager software (RevMan 2011). We used fixed‐effect meta‐analyses for combining data in the absence of significant heterogeneity if trials were sufficiently similar. When heterogeneity was found, we explored this by sensitivity analysis followed by random‐effects analysis.
Dichotomous data
For dichotomous data, we presented results as summary risk ratio (RR) with 95% confidence intervals (CIs).
Continuous data
For continuous data, we used the mean difference when outcomes were measured in the same way between trials. We used the standardised mean difference to combine trials that measured the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials for inclusion in this review. If we identify any cluster‐randomised trials in future updates of this review, we will include them in the analyses along with individually randomised trials. We will adjust their sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually randomised trials, we will synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a subgroup analysis to investigate the effects of the randomisation unit.
Dealing with missing data
For included studies, we noted levels of attrition. We have explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we have carried out analyses, as far as possible, on an intention‐to‐treat basis, that is we attempted to include all participants randomised to each group in the analyses, and all participants have been analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
In future updates of this review we will assess statistical heterogeneity in each meta‐analysis using the T2, I2 and Chi2 statistics. We will regard heterogeneity as substantial if I2 is greater than 30% and either T2 is greater than zero, or there is a low P value (less than 0.10) in the Chi2 test for heterogeneity.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually, and use formal tests for funnel plot asymmetry. For continuous outcomes we will use the test proposed by Egger 1997, and for dichotomous outcomes we will use the test proposed by Harbord 2006. If asymmetry is detected in any of these tests or is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We have carried out statistical analysis using Review Manager software (RevMan 2011). This review contains one included study.
In future updates of this review, we will carry out statistical analysis using the Review Manager software (RevMan 2011). We will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect: that is where trials are examining the same intervention, and the trials' populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful we will not combine trials.
If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% CIs, and the estimates of T2 and I2.
Subgroup analysis and investigation of heterogeneity
This review has one included study. Therefore, subgroup analysis and investigation of heterogeneity were not carried out.
In future updates of this review, if we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and, if it is, use random‐effects analysis to produce it.
We plan to carry out the following subgroup analyses.
Non‐selective COX inhibitors versus selective COX‐2 inhibitors.
Singleton versus multiple pregnancies.
Subgroup analysis will be restricted to primary outcomes.
For fixed‐effect inverse variance meta‐analyses we will assess differences between subgroups by interaction tests. For random‐effects and fixed‐effect meta‐analyses using methods other than inverse variance, we will assess differences between subgroups by inspection of the subgroups' CIs; non‐overlapping CIs indicate a statistically significant difference in treatment effect between the subgroups.
Sensitivity analysis
This review contains one included study. Therefore, planned sensitivity analysis was not carried out. In future updates, we plan to carry out sensitivity analysis to explore the effect of trial quality, only including studies that have been assessed as having adequate control of the potential for bias.
Results
Description of studies
Results of the search
We identified two potentially relevant studies. Mital 1992 was excluded because it was a quasi‐randomised trial (see Characteristics of excluded studies). One study was included to analysis (Groom 2005). The references of the two studies were searched for additional potential randomised trials, but none were found.
Included studies
We included one RCT in this review (Groom 2005). For more information see Characteristics of included studies.
The trial was undertaken at two teaching hospitals in London, UK, in singleton pregnant women with gestational ages of 16 to 26 weeks who were at high risk of preterm labour by at least one of the following criteria: at least two previous second trimester losses or early preterm deliveries < 30 weeks, one previous second trimester loss or early preterm birth < 30 weeks and cervical length ≤ 15 mm from 14 to 24 weeks, and cervical changes requiring cerclage in current pregnancy determined either by ultrasound criteria or clinically (rescue cerclage). Multiple pregnancies, previous allergy to NSAIDs and pregnant women with renal dysfunction were excluded. Ninety‐eight singleton pregnancies were eligible and stratified to ensure equal numbers with cerclage. They were then randomised to active treatment (rofecoxib 12.5 mg) or placebo once daily until 32 weeks of gestational age. Active treatment and placebo were prepared in identical gelatin‐covered capsules and issued by a pharmacist independent to the trial investigators. Main outcomes were fetal renal function and ductus arteriosus blood flow changes (change in amniotic fluid index and oligohydramnios, change in hourly fetal urine production rate, change in ductus arteriosus pulsatile index (PI) and maximum systolic velocity and discontinuation owing to change in renal function/ductal blood flow). Other outcomes included preterm birth rates and neonatal outcomes.
Excluded studies
One study was excluded (Mital 1992) because it was a quasi‐randomised trial (see Characteristics of excluded studies).
Risk of bias in included studies
(See Figure 1)
1.
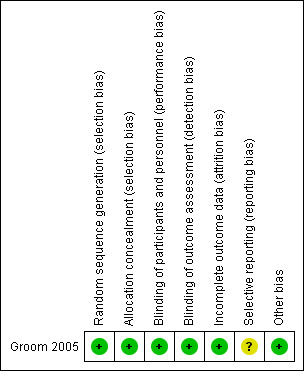
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
The study assigned participants into two groups using stratified randomisation. A computer‐generated randomisation programme was used. The participants were classified by risk for preterm birth and stratification was used to assigned participants who may have a higher risk for preterm birth (who received cervical cerclage) equally into study and control groups.
Blinding
For participants and investigators, rofecoxib and placebo were prepared by the pharmacist independently from trial investigators. There were no data for blinding outcome assessors but most of the outcomes were objective outcomes.
Incomplete outcome data
Outcomes of participants in the study were reported. There were no losses to follow‐up or drop‐outs.
Selective reporting
Although positive and negative sides of outcome data were reported, limitation for assessment of the protocol, selective reporting bias was addressed as unclear.
Other potential sources of bias
We found no other known biases. Baseline characteristics of the two groups in the included study were comparable.
Effects of interventions
This review included one good‐quality randomised controlled trial (involving 98 women) in which oral rofecoxib (once daily) was compared with a placebo control.
Primary outcomes
Preterm labour was not reported in the included study. However, administration of rofecoxib was associated with an increased risk for preterm birth when compared with placebo (RR 1.65; 95% CI 1.11 to 2.45; Analysis 1.1).
1.1. Analysis.
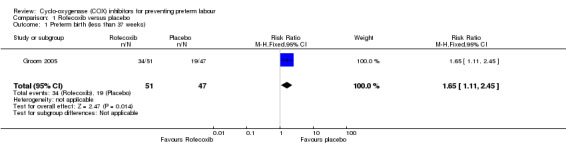
Comparison 1 Rofecoxib versus placebo, Outcome 1 Preterm birth (less than 37 weeks).
Secondary outcomes
For adverse effects, there was increased risk of preterm premature rupture of membranes (PPROM) with rofecoxib compared to placebo (RR 2.46; 95% CI 1.28 to 4.73; 1 trial; 98 women). There was also increased risk of oligohydramnios (RR 8.29; 95% CI 1.09 to 63.00; 1 trial; 98 women) but amniotic fluid returned to normal within one week after treatment. There was a significant increase in maximum systolic velocity and minimum diastolic velocity but no significant decrease of PI was observed. Difference in discontinuation of treatment before gestational age of 32 weeks was not significantly different between COX and placebo (RR 1.21; 95% CI 0.72 to 2.03) (Analysis 1.2).
1.2. Analysis.
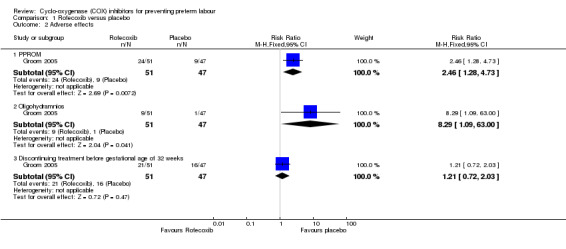
Comparison 1 Rofecoxib versus placebo, Outcome 2 Adverse effects.
Neonatal morbidities were reported. These included the need for ventilation, intraventricular haemorrhage, patent ductus arteriosus, mild tricuspid regurgitation/mild pulmonary hypertension, necrotising enterocolitis, renal calcification, nesidioblastosis, tetralogy of Fallot with 22q deletion, imperforated anus and chronic lung disease. There was no significant difference in neonatal morbidities between rofecoxib and placebo (Analysis 1.3).
1.3. Analysis.
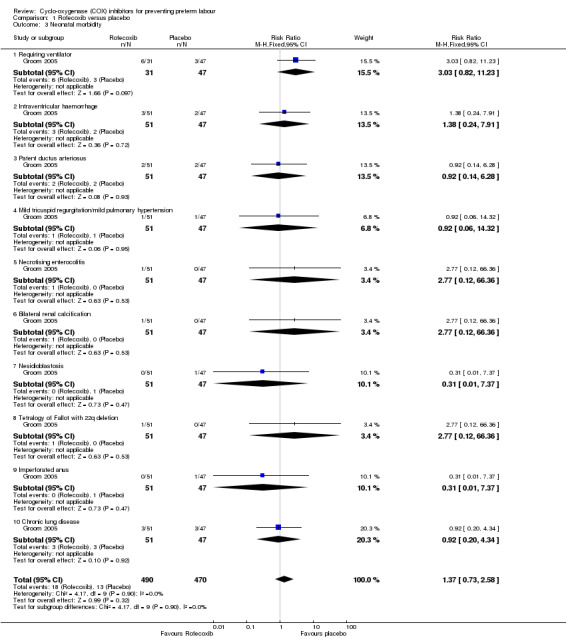
Comparison 1 Rofecoxib versus placebo, Outcome 3 Neonatal morbidity.
There was no significant difference in the number of neonates admitted to neonatal intensive care unit (RR 1.57; 95% CI 0.80 to 3.07; Analysis 1.4). There were no perinatal deaths in either group (Analysis 1.5).
1.4. Analysis.
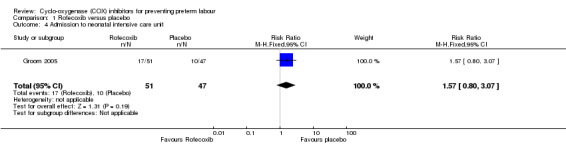
Comparison 1 Rofecoxib versus placebo, Outcome 4 Admission to neonatal intensive care unit.
1.5. Analysis.
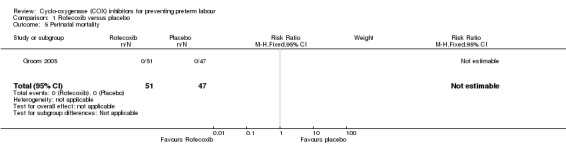
Comparison 1 Rofecoxib versus placebo, Outcome 5 Perinatal mortality.
Discussion
Summary of main results
We identified one randomised controlled trial including of 98 participants. There was no information about preterm labour. Use of rofecoxib was associated with an increased risk for preterm birth when compared with placebo. There were no statistical differences in neonatal morbidities or admission to neonatal intensive care unit. Rofecoxib was associated with increased risk of PPROM. It was also associated with a higher incidence of oligohydramnios and reduced fetal urine production but these effects were reversible when the treatment was stopped.
Overall completeness and applicability of evidence
We found no evidence to assess the effects of using COX inhibitors to prevent preterm labour. Limited evidence (one small study) suggests that COX inhibitors may increase the risk of preterm birth and more evidence is needed.
Quality of the evidence
We only identified one small study for inclusion in this review. However, the study was of good quality (low risk of bias).
Potential biases in the review process
We attempted to reduce bias wherever possible by having two review authors (Thirawut Khanprakob and Ussanee Sangkomkamhang) independently working on study selection and data extraction.
Agreements and disagreements with other studies or reviews
No other systematic reviews on the use of COX inhibitors for preventing preterm labour were identified.
Authors' conclusions
Implications for practice.
There was very little evidence about using COX inhibitors for preventing preterm labour. Therefore, there are inadequate data to make any recommendations about using COX inhibitors in clinical practice to prevent preterm labour.
Implications for research.
This review found no information to draw a conclusion of using COX inhibitors in preventing preterm labour and found no difference in neonatal morbidities when compared with placebo. There were disadvantages of using rofecoxib in terms of increasing risk of PPROM, reversible oligohydramnios and it may affect to fetal urine production. Future research should also include the follow‐up of babies to examine the short‐term and long‐term effects of the COX inhibitors.
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team) and the Group's Statistical Adviser.
Data and analyses
Comparison 1. Rofecoxib versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth (less than 37 weeks) | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.11, 2.45] |
| 2 Adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 PPROM | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [1.28, 4.73] |
| 2.2 Oligohydramnios | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.29 [1.09, 63.00] |
| 2.3 Discontinuing treatment before gestational age of 32 weeks | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.72, 2.03] |
| 3 Neonatal morbidity | 1 | 960 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.73, 2.58] |
| 3.1 Requiring ventilator | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.82, 11.23] |
| 3.2 Intraventricular haemorrhage | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.24, 7.91] |
| 3.3 Patent ductus arteriosus | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.14, 6.28] |
| 3.4 Mild tricuspid regurgitation/mild pulmonary hypertension | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.06, 14.32] |
| 3.5 Necrotising enterocolitis | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [0.12, 66.36] |
| 3.6 Bilateral renal calcification | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [0.12, 66.36] |
| 3.7 Nesidioblastosis | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.37] |
| 3.8 Tetralogy of Fallot with 22q deletion | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [0.12, 66.36] |
| 3.9 Imperforated anus | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.37] |
| 3.10 Chronic lung disease | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.20, 4.34] |
| 4 Admission to neonatal intensive care unit | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.80, 3.07] |
| 5 Perinatal mortality | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Groom 2005.
| Methods | Randomised controlled trial. | |
| Participants | 98 singleton pregnancies at high risk for preterm labour. Recruitment started from GA of 16 weeks up to 26 weeks. Setting: 2 teaching hospitals in London, UK. Inclusion criteria: at least 1 of the following:
Exclusion criteria: multiple pregnancy, previous allergy to NSAIDs, maternal renal dysfunction. |
|
| Interventions | Pregnant women in the study group received rofecoxib 12.5 mg once daily and in control group received placebo. Duration of treatment was not reported. Treatment completed at 32 weeks of GA. | |
| Outcomes |
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation to ensure equal numbers with cerclage assigned to each group was done using computer‐generated randomisation program. |
| Allocation concealment (selection bias) | Low risk | Treatment and placebo were prepared in identical gelatin‐covered capsules and issued by a pharmacist independent to the investigators. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment and placebo were prepared in identical gelatin‐covered capsules and issued by a pharmacist independent to the investigators. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Treatment and placebo were prepared in identical gelatin‐covered capsules and issued by a pharmacist independent to the investigators. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of all of the participants in the study were reported. There were no losses to follow‐up or drop‐outs. |
| Selective reporting (reporting bias) | Unclear risk | Both positive and negative outcomes were reported; however, we could not assess the protocol. |
| Other bias | Low risk | None known. Baseline characteristics of 2 groups were comparable. |
GA: gestational age; IVH: intraventricular haemorrhage; NEC: necrotising enterocolitis; NSAID: non‐steroidal anti‐inflammatory drug; PDA: patent ductus arteriosis; PPROM: preterm premature rupture of membranes; SCBU: special care baby unit.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Mital 1992 | The study used a quasi‐randomised method of allocation (alternation). |
Differences between protocol and review
The methods have been updated in accordance with the latest Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Potential risks of using COX inhibitors in pregnant women were added in Background section.
We have changed 'Types of participants' from "Pregnant women at gestational age less than 22 to 36 weeks' gestation, at risk of but not experiencing, preterm labour" to "Pregnant women at gestational age less than 36 weeks, at risk of but not experiencing, preterm labour". Thus removing any restriction on gestational age.
Contributions of authors
Thirawut Khanprakob (TK) registered the review title and drafted the protocol. Ussanee Sangkomkamhang (US), Pisake Lumbiganon (PL) and Malinee Laopaiboon (ML) revised and approved the final version of the protocol. TK drafted the review. US, PL and ML revised and approved the final version of the review.
Sources of support
Internal sources
Khon Kaen Hospital, Ministry of Public Health, Thailand.
Faculty of Medicine, Khon Kaen University, Thailand.
Faculty of Public Health, Khon Kaen University, Thailand.
External sources
Thailand Research Fund, Senior Research Scholar, Thailand.
Thai Cochrane Network, Thailand.
Declarations of interest
None known.
New
References
References to studies included in this review
Groom 2005 {published data only}
- Groom KM, Shennan AH, Jones BA, Seed P, Bennett PR. TOCOX ‐ a randomised, double‐blind, placebo‐controlled trial of rofecoxib (a COX‐2‐specific prostaglandin inhibitor) for the prevention of preterm delivery in women at high risk. BJOG: an international journal of obstetrics and gynaecology 2005;112(6):725‐30. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Mital 1992 {published data only (unpublished sought but not used)}
- Khuteta RP, Garg S, Bhargava A. Mefenamic acid in prevention of premature labour. 12th FIGO World Congress of Gynecology and Obstetrics; 1988 Oct 23‐28; Brazil. 1988:222.
- Mital P, Garg S, Khuteta RP, Khuteta S. Mefenamic acid in prevention of premature labour. Journal of the Royal Society of Health 1992;112(5):214‐6. [DOI] [PubMed] [Google Scholar]
Additional references
Arias 2003
- Arias E, Anderson RN, Kung HC, Murphy SL, Kochanek KD. Deaths: final data for 2001. National Vital Statistics Reports 2003;52(3):1‐115. [PubMed] [Google Scholar]
Beck 2010
- Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bulletin of the World Health Organization 2010;88(1):31‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blondel 2006
- Blondel B, Macfarlane A, Gissler M, Breart G, Zeitlin J. Preterm birth and multiple pregnancy in European countries participating in the PERISTAT project. BJOG: an international journal of obstetrics and gynaecology 2006;113(5):528‐35. [DOI] [PubMed] [Google Scholar]
Brodt‐Eppley 1999
- Brodt‐Eppley J, Myatt L. Prostaglandin receptors in lower segment myometrium during gestation and labor. Obstetrics & Gynecology 1999;93(1):89‐93. [DOI] [PubMed] [Google Scholar]
Cunningham 2005
- Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Gilstrap III L, Wenstrom KD. Williams Obstetrics. 22nd Edition. McGraw‐Hill, 2005. [Google Scholar]
Dawood 1993
- Dawood MY. Nonsteroidal antiinflammatory drugs and reproduction. American Journal of Obstetrics and Gynecology 1993;169(5):1255‐65. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibb 1998
- Gibb W. The role of prostaglandins in human parturition. Annals of Medicine 1998;30(3):235‐41. [DOI] [PubMed] [Google Scholar]
Gibb 2002
- Gibb W, Challis JR. Mechanisms of term and preterm birth. Journal of Obstetrics and Gynaecology Canada 2002;24(11):874‐83. [DOI] [PubMed] [Google Scholar]
Grigsby 2006
- Grigsby PL, Sooranna SR, Adu‐Amankwa B, Pitzer B, Brockman DE, Johnson MR, et al. Regional expression of prostaglandin E2 and F2alpha receptors in human myometrium, amnion, and choriodecidua with advancing gestation and labor. Biology of Reproduction 2006;75(2):297‐305. [DOI] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [DOI] [PubMed] [Google Scholar]
Henry 2005
- Henry PJ, D'Aprile A, Self G, Hong T, Mann TS. Inhibitors of prostaglandin transport and metabolism augment protease‐activated receptor‐2‐mediated increases in prostaglandin E2 levels and smooth muscle relaxation in mouse isolated trachea. Journal of Pharmacology and Experimental Therapeutics 2005;314(3):995‐1001. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hobel 2003
- Hobel C, Culhane J. Role of psychosocial and nutritional stress on poor pregnancy outcome. Journal of Nutrition 2003;133(5 Suppl 2):1709S‐1717S. [DOI] [PubMed] [Google Scholar]
Hoffman 1999
- Hoffman JD, Ward K. Genetic factors in preterm delivery. Obstetrical & Gynecological Survey 1999;54(3):203‐10. [DOI] [PubMed] [Google Scholar]
King 2005
- King J, Flenady V, Cole S, Thornton S. Cyclo‐oxygenase (COX) inhibitors for treating preterm labour. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001992.pub2] [DOI] [PubMed] [Google Scholar]
Kiss 2004
- Kiss H, Petricevic L, Husslein P. Prospective randomised controlled trial of an infection screening programme to reduce the rate of preterm delivery. BMJ 2004;329(7462):371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kliegman 2007
- Kliegman RM, Behrman RE, Jenson HB, Stanton BF. Nelson Textbook of Pediatrics. 18th Edition. Saunders Elsevier, 2007. [Google Scholar]
Kramer 1995
- Kramer MS, Coates AL, Michoud MC, Dagenais S, Hamilton EF, Papageorgiou A. Maternal anthropometry and idiopathic preterm labor. Obstetrics & Gynecology 1995;86(5):744‐8. [DOI] [PubMed] [Google Scholar]
Kurdi 2004
- Kurdi AM, Mesleh RA, Al‐Hakeem MM, Khashoggi TY, Khalifa HM. Multiple pregnancy and preterm labor. Saudi Medical Journal 2004;25(5):632‐7. [PubMed] [Google Scholar]
Loe 2005
Loudon 2003
- Loudon JA, Groom KM, Bennett PR. Prostaglandin inhibitors in preterm labour. Best Practice & Research. Clinical Obstetrics & Gynaecology 2003;17(5):731‐44. [DOI] [PubMed] [Google Scholar]
Luke 1995
- Luke B, Mamelle N, Keith L, Munoz F, Minogue J, Papiernik E, et al. The association between occupational factors and preterm birth: a United States nurses' study. Research Committee of the Association of Women's Health, Obstetric, and Neonatal Nurses. American Journal of Obstetrics and Gynecology 1995;173(3 Pt 1):849‐62. [DOI] [PubMed] [Google Scholar]
Meis 1995
- Meis PJ, Michielutte R, Peters TJ, Wells HB, Sands RE, Coles EC, et al. Factors associated with preterm birth in Cardiff, Wales. I. Univariable and multivariable analysis. American Journal of Obstetrics and Gynecology 1995;173(2):590‐6. [DOI] [PubMed] [Google Scholar]
Morken 2005
- Morken NH, Kallen K, Hagberg H, Jacobsson B. Preterm birth in Sweden 1973‐2001: rate, subgroups, and effect of changing patterns in multiple births, maternal age, and smoking. Acta Obstetricia et Gynecologica Scandinavica 2005;84(6):558‐65. [DOI] [PubMed] [Google Scholar]
Murphy 2007
- Murphy DJ. Epidemiology and environmental factors in preterm labour. Best Practice and Research: Clinical Obstetrics and Gynaecology 2007;21(5):773‐89. [DOI] [PubMed] [Google Scholar]
Myatt 2004
- Myatt L, Lye SJ. Expression, localization and function of prostaglandin receptors in myometrium. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2004;70(2):137‐48. [DOI] [PubMed] [Google Scholar]
Narumiya 1999
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiological Reviews 1999;79(4):1193‐226. [DOI] [PubMed] [Google Scholar]
Novy 1980
- Novy MJ, Liggins GC. Role of prostaglandins, prostacyclin, and thromboxanes in the physiologic control of the uterus and in parturition. Seminars in Perinatology 1980;4(1):45‐66. [PubMed] [Google Scholar]
Olson 1995
- Olson DM, Mijovic JE, Sadowsky DW. Control of human parturition. Seminars in Perinatology 1995;19(1):52‐63. [DOI] [PubMed] [Google Scholar]
Olson 2003
- Olson DM. The role of prostaglandins in the initiation of parturition. Best Practice & Research. Clinical Obstetrics & Gynaecology 2003;17(5):717‐30. [DOI] [PubMed] [Google Scholar]
Olson 2007
- Olson DM, Ammann C. Role of the prostaglandins in labour and prostaglandin receptor inhibitors in the prevention of preterm labour. Frontiers in Bioscience 2007;12:1329‐43. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Schieve 2000
- Schieve LA, Cogswell ME, Scanlon KS, Perry G, Ferre C, Blackmore‐Prince C, et al. Prepregnancy body mass index and pregnancy weight gain: associations with preterm delivery. The NMIHS Collaborative Study Group. Obstetrics & Gynecology 2000;96(2):194‐200. [DOI] [PubMed] [Google Scholar]
Smith 1996
- Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)‐1 and ‐2. Journal of Biological Chemistry 1996;271(52):33157‐60. [DOI] [PubMed] [Google Scholar]
Toppozada 1975
- Toppozada M, Gaafar A, Shaala S, Osman M. The relaxant property of local prostaglandin E‐2 on the non‐pregnant uterus ‐ a cyclic triphasic response. Prostaglandins 1975;9(3):475‐86. [DOI] [PubMed] [Google Scholar]
Tsai 2008
- Tsai HJ, Liu X, Mestan K, Yu Y, Zhang S, Fang Y, et al. Maternal cigarette smoking, metabolic gene polymorphisms, and preterm delivery: new insights on G&E interactions and pathogenic pathways. Human Genetics 2008;123:359‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ustun 2001
- Ustun C, Kocak I, Baris S, Uzel A, Saltik F. Subclinical chorioamnionitis as an etiologic factor in preterm deliveries. International Journal of Gynecology & Obstetrics 2001;72(2):109‐15. [DOI] [PubMed] [Google Scholar]
Vane 1998
- Vane JR, Botting RM. Mechanism of action of antiinflammatory drugs. International Journal of Tissue Reactions 1998;20(1):3‐15. [PubMed] [Google Scholar]
Vermillion 1997
Vermillion 2005
- ST, Robinson CJ. Antiprostaglandin drugs. Obstetrics and Gynecology Clinics of North America 2005;32(3):501‐17. [DOI] [PubMed] [Google Scholar]
Weiss 2004
- Weiss JL, Malone FD, Vidaver J, Ball RH, Nyberg DA, Comstock CH, et al. Threatened abortion: a risk factor for poor pregnancy outcome, a population‐based screening study. American Journal of Obstetrics and Gynecology 2004;190(3):745‐50. [DOI] [PubMed] [Google Scholar]
Williams 1994
- Williams SP, Dorn GW 2nd, Rapoport RM. Prostaglandin I2 mediates contraction and relaxation of vascular smooth muscle. American Journal of Physiology 1994;267(2 Pt 2):H796‐H803. [DOI] [PubMed] [Google Scholar]


