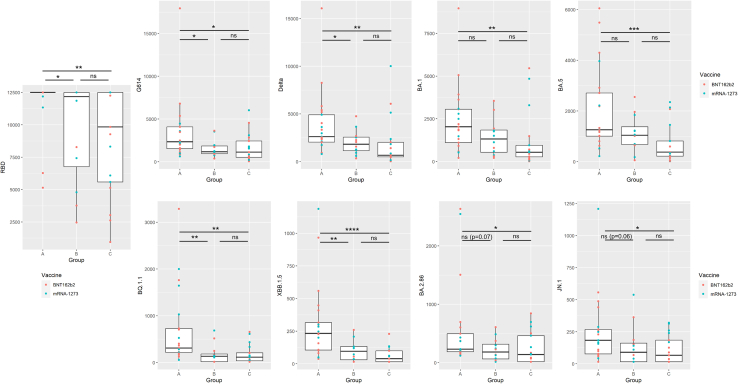
Figure 3.
Effect of breakthrough infections in the immunogenicity of the third vaccine dose
(Group A represent the results of participants with asymptomatic breakthrough infection (defined as any positive sample to SARS-CoV-2 nucleocapsid IgG during the study along with a negative sample at day 0).
Group B represent participants asymptomatically infected before the third dose (defined as subjects with a positive sample to anti-nucleocapsid IgG at day 0).
Group C represent participants who remained Nc- throughput the study period. Levels of immunoglobulins are reported in BAU/ml and neutralizing activity in neutralizing titer 50 (NT50). Data are represented as median and interquartile range. Ns: p > 0.05; ∗p ≤ 0.05; ∗∗p ≤ 0.01; ∗∗∗p ≤ 0.001; ∗∗∗∗p ≤ 0.0001.