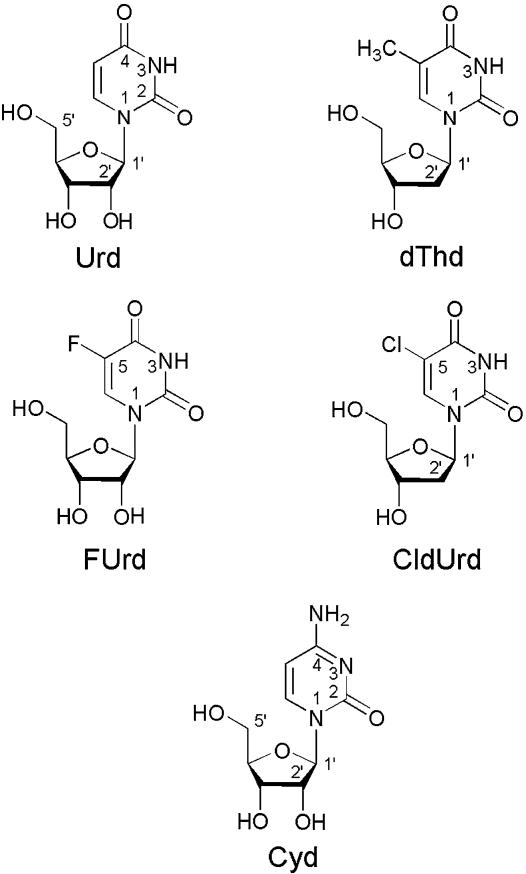
Fig. 1.
Chemical structures of Urd, dThd (2′-deoxy-5-methyluridine), FUrd, and CldUrd, as well as of Cyd in their dominating anti conformation (2, 18). For the anti–syn barrier, see ref. 12. The Urds are abbreviated as U and in the (N3)-deprotonated, anionic form as (U–H)– (U minus H); of course, the resulting negative charge at N3 can be delocalized in part to the neighboring carbonyl-oxygens.