Tuberculosis (TB) is responsible for roughly one-third of HIV-associated deaths among people with HIV (PWH). Despite the known risk among this population, only 56% (456 385) of an estimated 815 000 people with TB/HIV globally received a TB diagnosis and were reported in 2019 [1]. To close this gap, the WHO recommends screening PWH at every clinical encounter for cough of any duration, fever, weight loss and night sweats, followed by sputum testing with a WHO-recommended rapid diagnostic test when symptoms are present. Ruling out active disease is also crucial for safely initiating TB preventive treatment (TPT), which is a critical component of care for PWH. The U.S. President’s Emergency Plan for AIDS Relief (PEPFAR), the world’s largest HIV programme that provides antiretroviral therapy (ART) to 17.4 million people (roughly 65% of all PWH receiving ART), aligns with WHO guidance regarding TB screening [1,2]. We assess TB screening among ART patients in PEPFAR-supported sites and review existing literature on TB screening among PWH.
In 2020, PEPFAR-supported sites from 32 countries reported TB screening and treatment initiation data for 17.1 million ART patients. African countries accounted for 96% of these patients. Overall, 78% of ART patients were screened for TB at least once in the most recent 6-month period, and 2.1% of these screened positive for one or more symptoms. Among those who screened positive, 23% initiated TB treatment (0.4% of all ART patients). Among new ART patients, 6.2% and 3.1% screened positive for a TB symptom and initiated TB treatment, respectively; among existing ART patients, 1.9% screened positive for a TB symptom, and 0.3% initiated TB treatment. These proportions have decreased in 2020 compared to previous years (Fig. 1) [2].
Fig. 1.
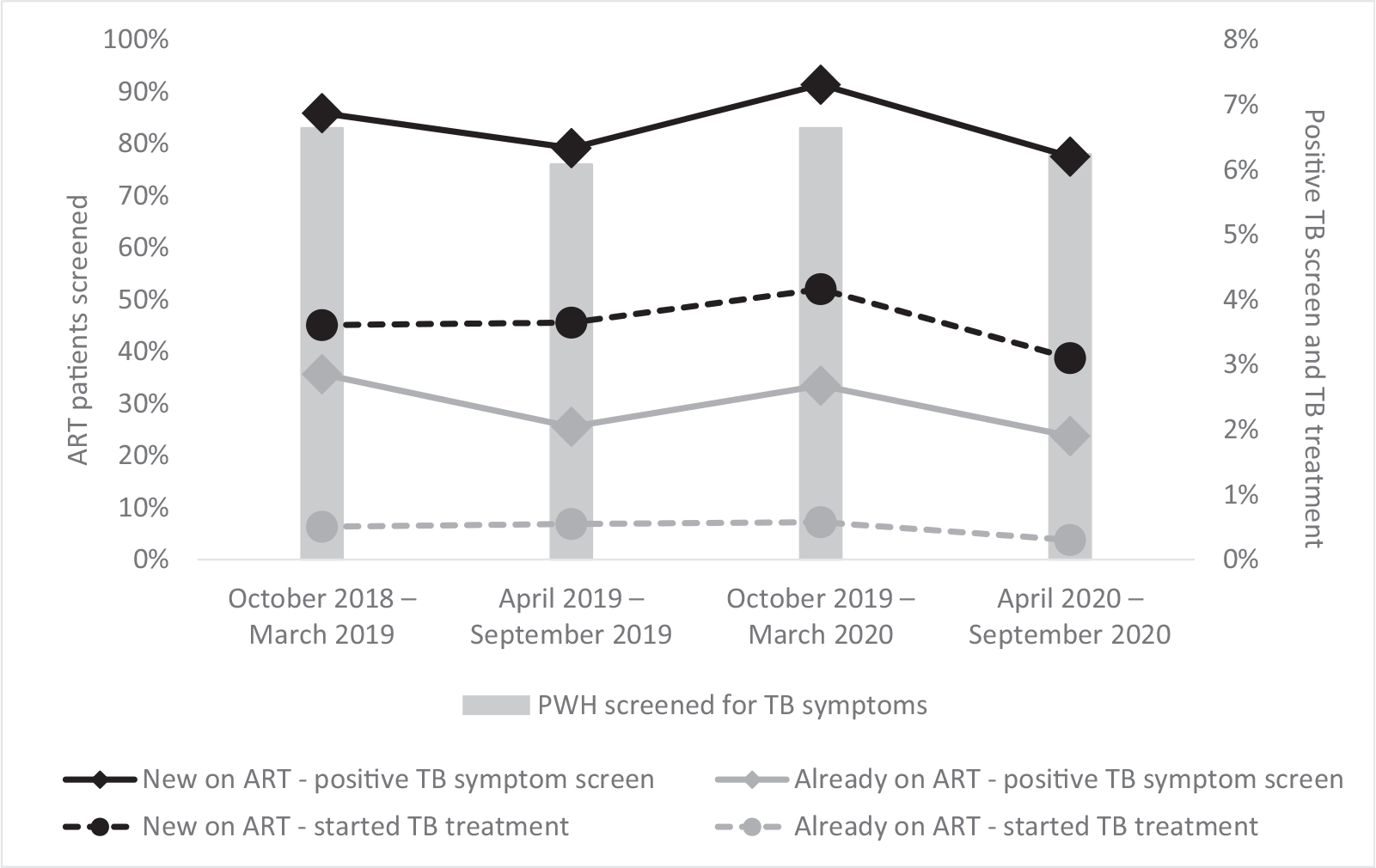
Pooled trends in percentage antiretroviral patients screened for tuberculosis, positive tuberculosis symptom screens and tuberculosis treatment initiations in US President Emergency Plan for AIDS Relief (PEPFAR)-supported sites.
Among PWH, expected incidence of TB reasonably varies depending on the dynamics of local TB and HIV epidemics; lower CD4+ cell count is also associated with a higher risk for TB [3]. A recent meta-analysis evaluating the WHO four-symptom screen among ART-naive PWH reported the positive screening yield from 12 studies totalling 8070 individuals throughout Africa. Among these pooled participants, 67% (5382) had a positive symptom screen, and 12.8% (1029) had active TB disease [4]. Median CD4+ cell count ranged from 127 to 394 cells/μl; among the highest study median CD4+ cell counts reported were 343 and 306 cells/μl, which were associated with 53% and 66% TB symptom positivity, respectively. Nine articles within this review included a combination of new and existing ART patients for a total of 7357 pooled PWH. Among these, 36.6% (2693) had a positive result for four-symptom screening, and 7.2% (530) had active TB [4]. Studies reporting adult CD4+ cell counts for those receiving ART ranged from 365 to 439 cells/μl, with an associated 30–44% screening positive for TB symptoms [4].
Broader adoption of the ‘test and treat’ approach, which promotes ART initiation regardless of CD4+ cell count, may explain some discrepancies between findings from these studies and recent data. WHO reported that worldwide CD4+ cell count at ART initiation increased from 133 to 272 between the years 2006 and 2014; increases are reported in all regions of Africa, where median CD4+ cell count at initiation was most recently reported to be between 225 (West Africa) and 350 cells/μl (central Africa) [1,5]. Duration of HIV treatment also influences TB risk; a recent analysis including data from over 45 000 individuals found that risk of developing active TB decreased by more than 50% over the first 3 years of ART; decreasing risk was observed across all CD4+ cell count categories [6]. In one study of 196 patients receiving ART for a median of 8 months, 83% (162) had a positive four-symptom screen, and 10.2% (20) had active TB disease [7]. Another study of 522 PWH receiving ART for a median duration of 2.2 years found 45% (233) had a positive four-symptom screen, and 6% (31) had active TB disease [8].
Given the evidence, we estimate that TB screening should yield a higher proportion of positive screens than what is observed at PEPFAR-supported sites. Although the proportion of PWH screened for TB is high, the low proportion of PWH with positive screening results implies deficits in screening. COVID-19 is threatening progress against HIV and TB, with the greatest losses predicted from delayed TB detection [9]. Symptom screening is an effective and cost-efficient way to detect TB disease among patients who are already accessing health systems [10]. Simple interventions such as feedback to clinicians after an audit have been promising in improving symptom screening [10]. Prioritizing continuity of TB screening services and high-quality screening across all models of care could help provide lifesaving TB treatment and TB preventive therapy worldwide.
Acknowledgements
M. Peterson contributed to literature review, data analysis, data interpretation, writing and figures.
N.S. Shah contributed to the analysis plan, literature review and writing.
S.E. Jeffcoat-Smith contributed to the literature review and writing.
C. Nichols contributed to the data analysis and data interpretation.
R. Fukunaga and T. Al-Samarrai contributed to the data interpretation and writing.
A. MacNeil contributed to the analysis plan and writing.
Data collection and analysis have been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies.
This project was approved by the CDC Center for Global Health Office of the Associate Director for Science/Laboratory Science.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.World Health Organization, Global Tuberculosis Report 2020, in Licence: CC BY-NC-SA 3.0 IGO. Geneva; WHO: 2020. [Google Scholar]
- 2.PEPFAR Monitoring, Evaluation, and Reporting Dataset, Operating Unit by Implementing Mechanism, in Monitoring, Evaluation, and Reporting Dataset. Washington, DC; PEPFAR; 2020. [Google Scholar]
- 3.Ellis PK, Martin WJ, Dodd PJ. CD4 count and tuberculosis risk in HIV-positive adults not on ART: a systematic review and meta-analysis. PeerJ 2017; 5:e4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamada Y, Lujan J, Schenkel K, Ford N, Getahun H. Sensitivity and specificity of WHO’s recommended four-symptom screening rule for tuberculosis in people living with HIV: a systematic review and meta-analysis. Lancet HIV 2018; 5:e515–e523. [DOI] [PubMed] [Google Scholar]
- 5.IeDEA and COHERE Cohort Collaborations. Global trends in CD4 cell count at the start of antiretroviral therapy: collaborative study of treatment programs. Clin Infect Dis 2018; 66: 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mupfumi L, Moyo S, Shin SS, Wang Q, Zetola N, Molebatsi K, et al. High incidence of tuberculosis in the first year of antiretroviral therapy in the Botswana National antiretroviral therapy programme between 2011 and 2015. AIDS 2019; 33:2415–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kufa T, Mngomezulu V, Charalambous S, Hanifa Y, Fielding K, Grant AD, et al. Undiagnosed tuberculosis among HIV clinic attendees: association with antiretroviral therapy and implications for intensified case finding, isoniazid preventive therapy, and infection control. J Acquir Immune Defic Syndr 2012; 60:e22–e28. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad Khan F, Verkuijl S, Parrish A, Chikwava F, Ntumy R, El-Sadr W, Howard AA. Performance of symptom-based tuberculosis screening among people living with HIV: not as great as hoped. AIDS 2014; 28:1463–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hogan AB, Jewell BL, Sherrard-Smith E, Vesga JF, Watson OJ, Whittaker C, et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middleincome countries: a modelling study. Lancet Glob Health 2020; 8:e1132–e1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjerrum S, Bonsu F, Hanson-Nortey NN, Kenu E, Johansen IS, Andersen AB, et al. Tuberculosis screening in patients with HIV: use of audit and feedback to improve quality of care in Ghana. Glob Health Action 2016; 9:32390. [DOI] [PMC free article] [PubMed] [Google Scholar]


