TABLE 2.
Levofloxacin pharmacokinetic parameters relative to MRSA 8043 and MRSA 8282 MICs and MPCs during in vitro system experimentsa
| Simulation (Cavg ss)b | MRSA strain | Parameter during first 24 h
|
Steady-state parameter
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
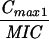 |
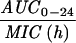 |
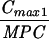 |
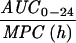 |
T0-24 MSW | 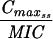 |
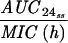 |
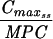 |
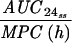 |
T24ss MSW | ||
| 125 mg every 24 h (0.68 μg/ml) | 8043 | 2.66 | 28.1 | 0.80 | 8.46 | 47.5 | 3.22 | 32.9 | 0.97 | 9.90 | 53.1 |
| 8282 | 2.64 | 28.2 | 0.72 | 7.70 | 46.1 | 3.05 | 32.4 | 0.83 | 8.84 | 57.5 | |
| 250 mg every 24 h (1.33 μg/ml) | 8043 | 5.48 | 56.8 | 1.65 | 17.1 | 55.7 | 6.17 | 63.3 | 1.86 | 19.1 | 56.0 |
| 8282 | 5.33 | 56.6 | 1.46 | 15.5 | 58.9 | 5.99 | 64.1 | 1.64 | 17.5 | 62.2 | |
| 500 mg every 24 h (2.62 μg/ml) | 8043 | 10.7 | 113 | 3.22 | 34.0 | 41.9 | 12.1 | 127 | 3.64 | 38.2 | 37.0 |
| 8282 | 10.6 | 114 | 2.89 | 31.1 | 45.9 | 11.8 | 125 | 3.22 | 34.2 | 41.7 | |
| 750 mg every 24 h (3.93 μg/ml) | 8043 | 16.1 | 164 | 4.85 | 49.5 | 22.2 | 18.9 | 188 | 5.69 | 56.8 | 19.6 |
| 8282 | 16.2 | 167 | 4.43 | 45.7 | 28.3 | 18.5 | 188 | 5.05 | 51.5 | 24.4 | |
Expressed as the means of results of two separate experiments. Cmax1, Cmax attained with the first dose; Cmax ss, Cmax at steady state; T0-24 MSW, the percentage of time levofloxacin was in the MSW during the first 24-h period; T24ssMSW, the percentage of time levofloxacin was in the MSW during a 24-h period at steady state.
Cavg ss for the simulated dosage regimens are expressed as the means of results of two separate experiments.
