Abstract
The inhibitory effects of hinokitiol (β-thujaplicin) on Chlamydia trachomatis D/UW-3/Cx were shown by MIC, minimum lethal concentration (MLC), and preinoculation minimal microbicidal concentration assays using HeLa 229 cells. The MIC and the MLC were both 32 μg/ml. Further evaluation of hinokitiol as a topical agent against C. trachomatis is warranted.
Chlamydia trachomatis is the most frequent cause of sexually transmitted diseases (4). Considering possible drug resistance (9, 14, 15), the availability of several approaches such as topical microbicides (2, 3, 10, 11, 16) to control chlamydial infections would be useful. Hinokitiol (β-thujaplicin) is a tropolone-related compound that is present in the heartwood of several cupressaceae, such as Chamaecyparis obtusa Sieb. et Zucc and Thuja plicata D. Don. Hinokitiol has antimicrobial activity against several microorganisms (6), such as influenza virus (12), Staphylococcus aureus, Staphylococcus epidermidis (1, 7, 8, 13), and Schistosoma mansoni (5). The objective of this study was to clarify the in vitro inhibitory effects of hinokitiol on C. trachomatis.
Serovar D of C. trachomatis D/UW-3/Cx and HeLa 229 cells were used in this study. Hinokitiol (β-thujaplicin; Fig. 1) was kindly provided by Takasago Int. Co. (Tokyo, Japan). MIC, minimum lethal concentration (MLC), and preinoculation minimal microbicidal concentration (MCC) assays were used to determine the susceptibility of C. trachomatis to hinokitiol. The inclusion count (% of control) was determined by the following formula: inclusion-forming units (IFU) in test sample ÷ IFU in control × 100%.
FIG. 1.
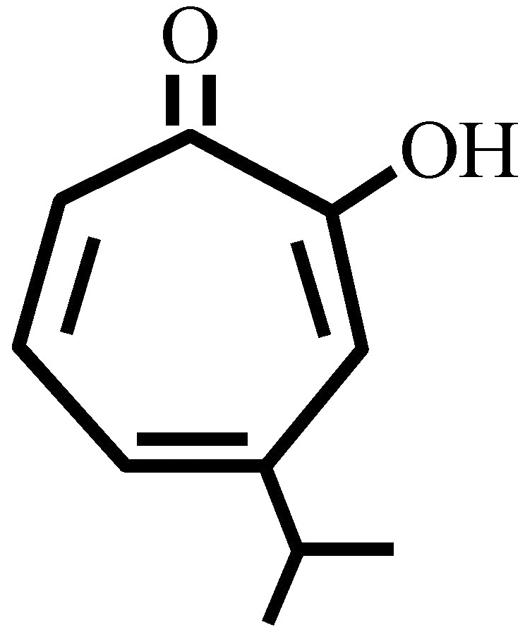
Structural formula of hinokitiol (β-thujaplicin).
After being inoculated onto a monolayer of HeLa 229 cells, 1 × 105 IFU per ml of C. trachomatis strains was incubated for 2 h at 37°C. Then the inocula were aspirated and 1 ml of the culture medium (Eagle's minimum essential medium containing 10% fetal calf serum and 1 μg per ml of cycloheximide) containing serially diluted hinokitiol was replaced. After incubation in 5% CO2 at 37°C for 72 h, the cultures were fixed with methanol and stained with fluorescein isothiocyanate-conjugated monoclonal antibody specific for C. trachomatis (Syva MicroTrak; Syva Company, San Jose, CA). Inclusions were counted by fluorescence microscopy, and the condition of cells was noted. The MIC was defined as the lowest concentration at which no inclusions were found. The MLC was defined as the lowest concentration of hinokitiol that resulted in no inclusions after passage with hinokitiol-free culture medium.
The MCC assay is similar to the preinoculation method previously described by Lampe et al. (11). In brief, 1.0 × 104 IFU of C. trachomatis strain was incubated with serial dilutions of hinokitiol at 37°C for 60 min. The mixture was inoculated onto HeLa 229 cells and incubated at 37°C for 2 h. After the inocula were removed, culture medium without hinokitiol was added and incubated for 72 h. The preinoculation MCC was defined as the lowest concentration of hinokitiol at which no inclusions were found.
To determine whether hinokitiol had any effect on HeLa 229 cells before infection with C. trachomatis, we added 1 ml of serially diluted hinokitiol to HeLa 229 cells and incubated them at 37°C for 60 min. Hinokitiol was then removed, and C. trachomatis was inoculated as described above.
The toxic effect of hinokitiol on HeLa 229 cells was evaluated by a cell counting kit (Dojindo Laboratory Co., Ltd., Kumamoto, Japan), incorporating a colorimetric assay for cell proliferation and viability using 2-(4-lodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monoso-dium salt (WST-1). Cell activity was determined in accordance with the manufacturer's instructions. In brief, serial dilutions of hinokitiol were dispensed onto microtiter plates containing HeLa 229 cells and incubated at 37°C for 72 h. After the hinokitiol solution was removed, cell activity was counted and the condition of the monolayer cells stained with Giemsa stain was assessed at ×400 magnification.
The results of MIC, MLC, and MCC assays are shown in Table 1. The MIC and the MLC of hinokitiol for C. trachomatis were both 32 μg/ml. The number of C. trachomatis inclusions in the MCC assay decreased with increasing concentrations of hinokitiol. At a concentration of 128 μg/ml of hinokitiol, the number of inclusions was 75% of that in control HeLa 229 cells. However, the number of inclusions decreased only 2% when HeLa 229 cells were preincubated with 32 μg/ml of hinokitiol prior to infection with C. trachomatis (Table 1). No morphological changes were observed in the HeLa 229 cells.
TABLE 1.
Inhibitory effect of hinokitiol on serovar D/UW-3/Cx of C. trachomatis
| Assay | Mean ± SD inclusion count (% of control)a after exposure to hinokitiol concn (μg/ml) of:
|
||||
|---|---|---|---|---|---|
| 2 | 4 | 8 | 16 | 32 | |
| MIC | 81 ± 6 | 36 ± 1 | 28 ± 3 | 12 ± 3 | 0 ± 0 |
| MLC | NDc | 96 ± 12 | 56 ± 9 | 5 ± 2 | 0 ± 0 |
| MCC | ND | ND | 82 ± 1 | 74 ± 5 | 70 ± 4 |
| Cellb | ND | ND | 99 ± 5 | 100 ± 3 | 98 ± 3 |
Inclusion count (% of control) = IFU in test sample ÷ IFU in control × 100%.
Cell, preincubation with HeLa 229 cells prior to infection.
ND, not done.
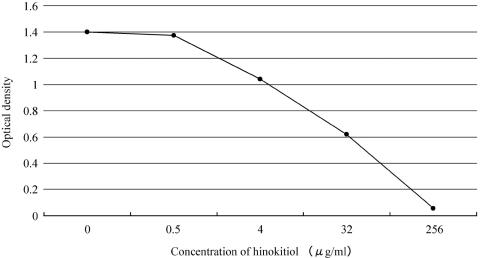
The cytotoxic effects of hinokitiol on HeLa 229 cells as assessed by the WST-1 assay are shown in Fig. 2. Cell activity decreased with increasing concentrations of hinokitiol. Although no decrease in activity was observed at hinokitiol concentrations of less than 1 μg/ml, activity decreased to 50% of the control value at 32 μg/ml of hinokitiol; however, no morphological changes were apparent by Giemsa staining at 32 μg/ml.
FIG. 2.
Cytotoxic effects of hinokitiol on HeLa 229 cells, as assessed by the WST-1 assay. HeLa 229 cells were incubated with serial concentrations of hinokitiol in 5% CO2 at 37°C for 72 h. Cell activity decreased to 50% at a concentration of 32 μg/ml of hinokitiol. However, morphological effects were not observed at this concentration by Giemsa staining.
Results of the MIC assay demonstrate that hinokitiol has an in vitro inhibitory effect on C. trachomatis at a concentration of 32 μg/ml. However, the concentration of hinokitiol required for complete inhibition of chlamydial proliferation is relatively high in comparison to the MICs of antibiotics such as tetracyclines, macrolides, and fluoroquinolones. The MICs of hinokitiol for S. aureus were reported to vary as follows: 1.56 to 3.13 μg/ml (1), 8.2 μg/ml (7), and 6.25 to 12.5 μg/ml (8). For clinical applications, therefore, hinokitiol could be used topically.
Our MIC and MCC assays both showed that hinokitiol has antimicrobial activity against C. trachomatis, indicating inhibitory effects both outside and inside HeLa 229 cells. This implies the potential clinical utility of hinokitiol for the prevention of C. trachomatis infection. We suggest that continuous or repeated topical application of hinokitiol as an appropriate gel or jelly would be helpful in inactivating C. trachomatis.
Although changes in the morphology of HeLa 229 cells were not observed at 32 μg/ml of hinokitiol, cell activity decreased at hinokitiol concentrations of more than 4 μg/ml after an incubation of 72 h. In addition, preincubation of HeLa 229 monolayers with hinokitiol for 60 min had little effect on the number of chlamydial inclusions and no morphological changes were observed. The most appropriate concentration of hinokitiol for application to a mucous membrane remains to be determined.
In conclusion, hinokitiol has inhibitory effects on C. trachomatis and may be clinically useful as a topical drug; however, the mechanisms of its inhibitory activity should be investigated further.
Acknowledgments
This work was supported by a grant from the Research Fund of the Saitama Medical School.
REFERENCES
- 1.Arima, Y., Y. Nakai, R. Hayakawa, and T. Nishino. 2003. Antibacterial effect of beta-thujaplicin on staphylococci isolated from atopic dermatitis: relationship between changes in the number of viable bacterial cells and clinical improvement in an eczematous lesion of atopic dermatitis. J. Antimicrob. Chemother. 51:113-122. [DOI] [PubMed] [Google Scholar]
- 2.Bélec, L., C. Tevi-Benissan, A. Bianchi, S. Cotigny, M. Beumont-Mauviel, A. Si-Mohamed, and J.-E. Malkin. 2000. In vitro inactivation of Chlamydia trachomatis and of a panel of DNA (HSV-2, CMV, adenovirus, BK virus) and RNA (RSV, enterovirus) viruses by the spermicide benzalkonium chloride. J. Antimicrob. Chemother. 46:685-693. [DOI] [PubMed] [Google Scholar]
- 3.Benes, S., and W. M. McCormack. 1985. Inhibition of growth of Chlamydia trachomatis by nonoxynol-9 in vitro. Antimicrob. Agents Chemother. 27:724-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1997. Chlamydia trachomatis genital infections—United States, 1995. Morb. Mortal. Wkly. Rep. 46:193-198. [PubMed] [Google Scholar]
- 5.Chisty, M. M., M. Nargis, T. Inaba, K. Ishita, A. Osanai, and H. Kamiya. 2004. Transmission electron microscopy of Schistosoma mansoni cercariae treated with hinokitiol (beta-thujaplicin), a compound for potential skin application against cercarial penetration. Tohoku J. Exp. Med. 202:63-67. [DOI] [PubMed] [Google Scholar]
- 6.Erdtman, H. and J. Gripenberg. 1948. Antibiotic substances from the heart wood of Thuja plicata Don. Nature 161:719. [DOI] [PubMed] [Google Scholar]
- 7.Inamori, Y., C. Muro, E. Sajima, M. Katagiri, Y. Okamoto, H. Tanaka, Y. Sakagami, and H. Tsujibo. 1997. Biological activity of purpurogallin. Biosci. Biotech. Biochem. 61:890-892. [DOI] [PubMed] [Google Scholar]
- 8.Inamori, Y., S. Shinohara, H. Tsujibo, T. Okabe, Y. Morita, Y. Sakagami, Y. Kumeda, and N. Ishida. 1999. Antimicrobial activity and metalloprotease inhibition of hinokitiol-related compounds, the constituents of Thujopsis dolabrata S. and Z. hondai MAK. Biol. Pharm. Bull. 22:990-993. [DOI] [PubMed] [Google Scholar]
- 9.Jones, R. B., B. van del Pol, D. H. Martin, and M. K. Shepard. 1990. Partial characterization of Chlamydia trachomatis isolates resistant to multiple antibiotics. J. Infect. Dis. 162:1309-1315. [DOI] [PubMed] [Google Scholar]
- 10.Kelly, J. P., R. B. Reynolds, S. Stagno, W. C. Louv, and W. J. Alexander. 1985. In vitro activity of the spermicide nonoxynol-9 against Chlamydia trachomatis. Antimicrob. Agents Chemother. 27:760-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lampe, M. F., L. M. Ballweber, and W. E. Stamm. 1998. Susceptibility of Chlamydia trachomatis to chlorhexidine gluconate gel. Antimicrob. Agents Chemother. 42:1726-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyamoto, D., Y. Kusagaya, N. Endo, A. Sometani, S. Takeo, T. Suzuki, Y. Arima, K. Nakajima, and Y. Suzuki. 1998. Thujaplicin-copper chelates inhibit replication of human influenza viruses. Antiviral Res. 39:89-100. [DOI] [PubMed] [Google Scholar]
- 13.Saeki, Y., Y. Ito, M. Shibata, Y. Sato, K. Okuda, and I. Takazoe. 1989. Antimicrobial action of natural substances on oral bacteria. Bull. Tokyo Dent. Coll. 30:129-135. [PubMed] [Google Scholar]
- 14.Somani, J., V. B. Bhullar, K. A. Workowski, C. E. Farshy, and C. M. Black. 2000. Multiple drug-resistant Chlamydia trachomatis associated with clinical treatment failure. J. Infect. Dis. 181:1421-1427. [DOI] [PubMed] [Google Scholar]
- 15.Stamm, W. E. 2000. Potential for antimicrobial resistance in Chlamydia pneumoniae. J. Infect. Dis. 181(Suppl. 1):S456-S459. [DOI] [PubMed] [Google Scholar]
- 16.Yamazaki, T., M. Inoue, N. Sasaki, T. Hagiwara, T. Kishimoto, S. Shiga, M. Ogawa, Y. Hara, and T. Matsumoto. 2003. In vitro inhibitory effects of tea polyphenols on the proliferation of Chlamydia trachomatis and Chlamydia pneumoniae. Jpn. J. Infect. Dis. 56:143-145. [PubMed] [Google Scholar]