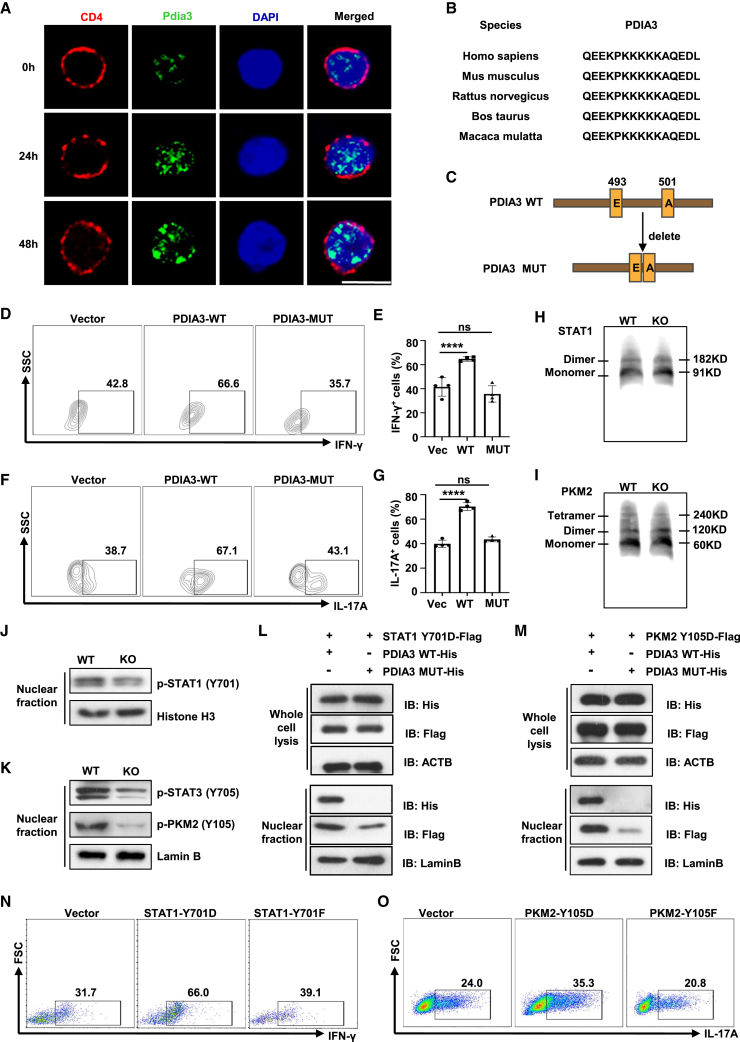
Figure 7.
PDIA3 facilitates STAT1 and PKM2 nuclear import to favor the effector T cell program
(A) Confocal microscopy of PDIA3 (green) in Tn cells stimulated with anti-CD3/CD28 antibodies at the indicated time points. Scale bar, 10 μm. (B) Alignment of NLS sequences of PDIA3 among different species. (C) PDIA3 mutation with deletion of its NLS was established based on the NLS_mapper prediction. (D–G) Tn cells were transduced with vector, PDIA3-WT, or PDIA3-MUT retroviruses under Th1- and Th17-polarizing conditions. Flow cytometry data for IFN-γ (D) and IL-17A (F) staining, and the percentages of IFN-γ+ Th1 (E) and IL-17A+ Th17 (G) cells. (H and I) WT and KO CD4 T cells were exposed to IL-12 (H) or IL-6 (I) for 6 h and then analyzed by native PAGE. (J) Western blot analysis of p-STAT1 in nuclear fraction from WT and KO CD4 T cells following IL-12 stimulation for 30 min. (K) Western blot analysis of p-PKM2 and p-STAT3 in the nuclear fraction from WT and KO CD4 T cells following IL-6 stimulation for 30 min. (L) HEK 293T cells were transfected with a Flag tag-labeled STAT1-Y701D plasmid combined with a His tag-labeled WT Pdia3 plasmid (PDIA3 WT-His) or a His tag-labeled mutant Pdia3 plasmid (PDIA3 MUT-His). Western blot analysis of PDIA3 and STAT1 in whole cell lysate and nuclear fraction, respectively. (M) HEK 293T cells were transfected with Flag tag-labeled PKM2-Y105D plasmid combined with PDIA3 WT-His or PDIA3 MUT-His, respectively. Western blot analysis of PDIA3 and PKM2 in whole cell lysate and nuclear fraction. (N) KO Tn cells were transduced with Vector, Retro-STAT1-Y701D or Retro-STAT1-Y701F and cultured under Th1 condition for 3 days. The frequencies of IFN-γ+ Th1 cells were shown in representative dot plots. (O) KO Tn cells were transduced with vector, Retro-PKM2-Y105D, or Retro-PKM2-Y105F and cultured under Th17 condition for 3 days. The frequencies of IL-17A+ Th17 cells are shown in representative dot plots. All in vitro studies were repeated at least three times.