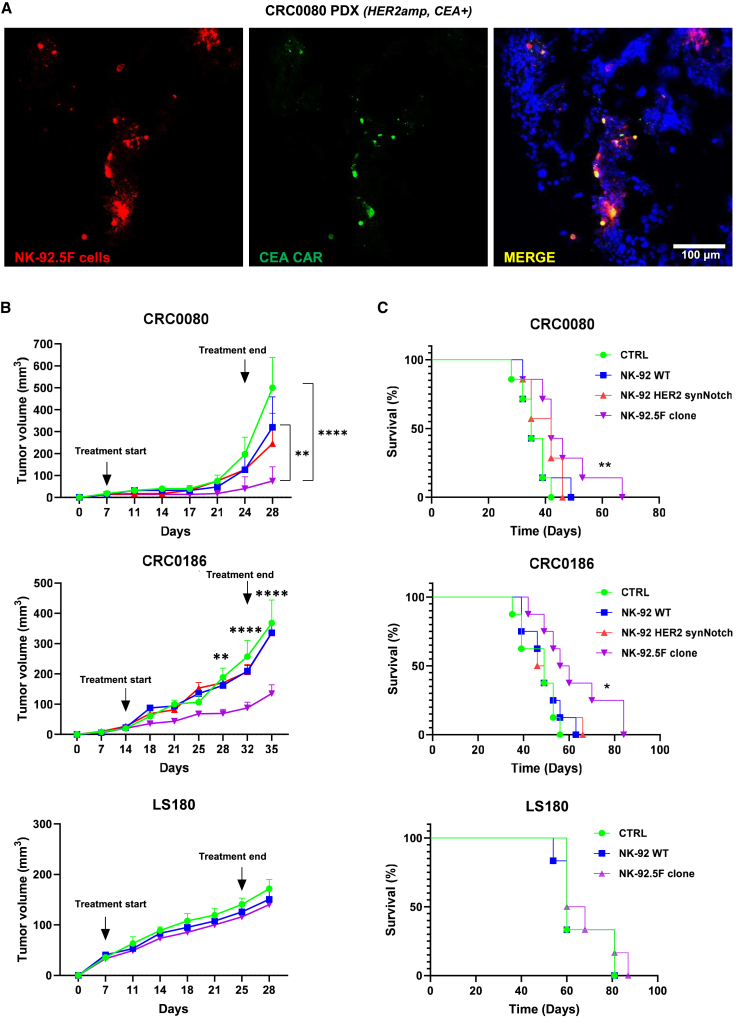
Figure 7.
NK-92.5F in vivo therapeutic efficacy
(A) Representative immunofluorescence images of CEA-CAR expression by the NK-92.5F clone after injection into CRC0080 xenograft tumors; the tumor was explanted 3 days after intratumoral inoculation of NK-92.5F (red: pkh26-labeled NK-92.5F cells; green: CEA-CAR antibody; blue: NucBlue-labeled cancer cells). Original magnification ×10; scale bars, 100 μm (two independent experiments). (B) In vivo growth of CRC0080, CRC0186, and LS180 xenografts treated with different NK-92 effectors, as indicated. We intravenously injected 5 × 106 irradiated effectors twice a week for a total of six injections. CTRL, PBS. Bars are SEM. Growth curves are stopped when the first mouse of the control cohort has reached the humane endpoint. Two-way ANOVA p-values: ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, ∗∗∗∗p ≤ 0.0001, between NK-92.5F clone vs. CTRL or WT NK-92 in CRC0080, and between NK-92.5F vs. all control cohorts in CRC0186. (C) Survival curves of mice implanted with CRC0080, CRC0186 or LS180 xenografts and intravenously injected with 5 × 106 NK-92 irradiated effector cells, as indicated. Statistical significance between CTRL and 5F clone was calculated by log rank test for trend. ∗p ≤ 0.05, ∗∗p ≤ 0.01.