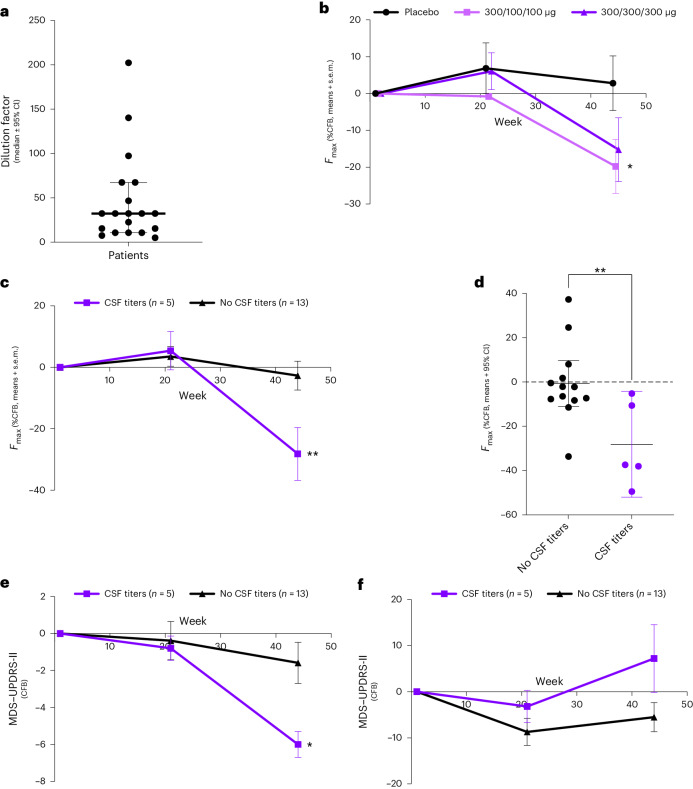
Fig. 3. Evaluation of αSyn seeds in CSF and MDS–UPDRS scores in patients with PD with and without CSF titers.
a, CSF samples collected before treatment (baseline) were serially diluted and tested in the αSyn-SAA (n = 20). Data presented are maximal dilution factor achieved before losing the signal in the αSyn-SAA; the bar represents median ± 95% CI. b, Maximum fluorescence (%CFB, mean ± s.e.m.) in CSF samples collected at baseline, week 21 and week 45 in patients treated with placebo (n = 6), UB-312 300/100/100 μg (n = 6, one patient provided CSF only at baseline and is excluded) or UB-312 300/300/300 μg (n = 6, one individual who was αSyn-SAA negative at baseline is excluded) showed a significant difference between placebo and 300/100/100 μg (two-way ANOVA, time × treatment interaction, P = 0.0343). c, A comparison of the maximum fluorescence (%CFB, mean ± s.e.m.) in CSF samples collected at baseline, week 21 and week 45 showed a significant difference (two-way ANOVA, time × treatment interaction, P = 0.0037) between individuals with (n = 5) versus without (n = 13) detectable antibody titers. d, Maximum fluorescence at EoS (%CFB means of three technical replicates per individual; bar represents median ± 95% CI) shows a significant between-group difference (two-tailed unpaired t-test, P = 0.0094). e, A comparison in CFB (mean ± s.e.m.) in MDS–UPDRS-II score showed a significant difference (unpaired t-test, time × group interaction, P = 0.016) between patients with (n = 5) versus without (n = 13) detectable CSF antibody titers. The subcomponent that showed the greatest difference in this change was ‘Getting out of bed, getting out of a car or standing up from a low chair’ (Supplementary Table 1). A two-way ANOVA with a mixed-effect model was followed by within-group analysis per Benjamini, Krieger and Yekitieli. f, A comparison in the CFB (mean ± s.e.m.) in MDS–UPDRS-III score between patients with (n = 5) versus without (n = 13) detectable CSF antibody titers. A two-way ANOVA was used as per e. In a–f, *P < 0.05 and **P < 0.01. CI, confidence interval.