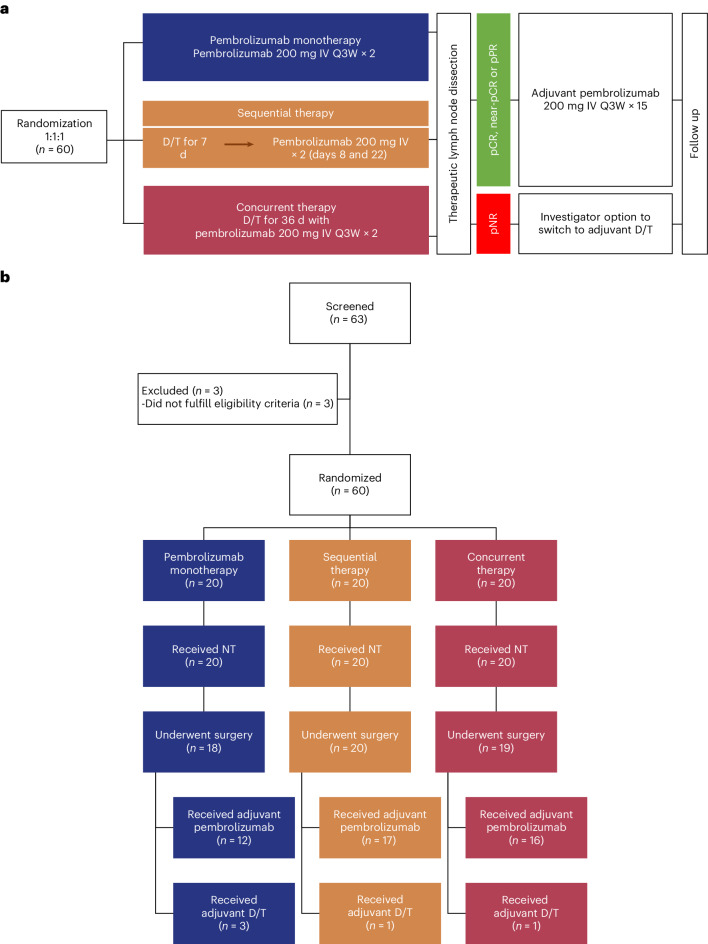
Fig. 1. Trial design and patient flow diagram.
a, Overview of the non-comparative randomized phase 2, open-label, three-arm NeoTrio trial (ClinicalTrials.gov registration: NCT02858921), with pathological response-directed adjuvant therapy. Dosing regimen for D/T was dabrafenib 150 mg PO BID plus trametinib 2 mg PO QD. b, Patient flow diagram. BID, twice per day; D/T, dabrafenib plus trametinib; NT, neoadjuvant therapy; PO, per oral; QD, once per day; Q3W, every three weeks.