Abstract
Variation in fermentation time may be an essential alternative to provide coffee beverages with different and unique sensory profiles. This work investigated the microbiological, chemical, and sensory changes in coffees submitted to different fermentation durations (0, 24, 48, 72, and 96 h). Self-induced anaerobiosis fermentation (SIAF) was used, and two treatments were performed: spontaneous fermentation and inoculation with S. cerevisiae CCMA0543. Microbiological analyses were performed, and the permanence of the inoculum was monitored. Chromatography (sugars, organic acids, and volatile compounds) was analyzed, and sensory analysis (temporal dominance of sensations - TDS) was performed. A total of 228 isolates were identified during spontaneous fermentation. The dominant bacteria and yeasts were Leuconostoc mesenteroides, Lactiplantibacillus plantarum, Staphylococcus warneri, Bacillus sp., Torulaspora delbrueckii, Hanseniaspora uvarum, and Meyerozyma caribbica. High concentrations of citric (18.67 mg.g− 1) and succinic (5.04 mg.g− 1) acids were detected at 96 h in SIAF fermentation. One hundred twenty-one volatile compounds were detected, but 22 were detected only in inoculated coffees. In spontaneous fermentation, 48 h of fermentation showed woody notes, while 72 h showed chestnuts. However, in the inoculated coffee, 72 h of fermentation showed high fruity dominance, and 96 h of fermentation was the only one with herbaceous notes. In addition, yeast inoculation increased the intensity of caramel notes in the first 48 h and increased the fruity flavor after 72 h of fermentation. Therefore, the type of fermentation (with or without inoculation) and the chosen fermentation time will depend on the sensorial profile the producer intends to obtain.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-024-01370-6.
Keywords: SIAF, Coffee fermentation, Chemical analysis, Microbiological analysis, Starter cultures
Introduction
Coffee is one of the world’s most prominent commodities, prepared from ground and roasted coffee beans. This beverage is widely consumed because it possesses stimulating properties and is a source of antioxidants [1–4]. Brazil is the largest coffee producer in the world, followed by Vietnam and Colombia. In 2023, the production estimate is 54.74 million processed bags [5].
The global coffee market is very dynamic. Consequently, the pursuit of coffee production with distinctive sensory profiles has captured the industry’s attention. Innovations in post-harvest techniques have been employed to ensure more efficient and high-quality fermentation processes. A pioneering method, Self-Induced Anaerobiosis Fermentation (SIAF), has been used to provide adequate microbiota performance. Pulped or natural coffee is fermented in covered polypropylene bioreactors with hermetic closure to prevent air entry or exit. This method ensures anaerobiosis by gradual O2 consumption and CO2 production. Consequently, the lactic acid bacteria and yeast populations increase, producing metabolites that favor the flavor and aroma of coffee beverages [6–8].
The diversity of coffee flavors is attributed to the microbiota present during fermentation, influenced by the region’s climatic conditions. Pectinolytic microorganisms, including Saccharomyces cerevisiae, Bacillus subtilis, Candida parapsilosis, Torulaspora delbrueckii, Pichia caribbica, and Pichia guilliermondii, have been selected as starter cultures and have yielded promising results in modulating the flavor of the coffee [9–12].
Saccharomyces cerevisiae has been widely used in several biotechnology processes due to its metabolic profile, such as fermentative metabolism, resistance to high concentrations of sugar and ethanol, and production of specific aromatic compounds, besides being considered safe (GRAS) [13]. This yeast is naturally present during coffee fermentation, and its ability to produce high concentrations of pectin lyase, polygalacturonase, and pectin methylesterase is essential to the process standardization [14, 15].
Among these strains, S. cerevisiae CCMA 0543, designated initially as UFLA YCN 727, has been used for coffee fermentation since 2014. It has shown potential as a starter culture to improve the sensory quality of coffee beverages [16, 17]. Ribeiro et al. [18, 19] showed that the addition of S. cerevisiae CCMA 0543 in fermentation under aerobic conditions enhanced coffee acidity, improving sensory results among the yeast strains in two coffee varieties: Ouro Amarelo and Mundo Novo. This strain was considered the most suitable inoculant due to its enhanced persistence and the number of volatile compounds produced during fermentation.
Martinez et al. [4] and Bressani et al. [20] used the same yeast strains in different inoculation methods in the Catuaí Amarelo variety. First, the yeasts were spread on the coffee, and then the coffee was kept in buckets for some time. This method favored the permanence of bacteria and yeasts during fermentation, and exotic sensory notes of cashews were strongly associated with the yeast strain and the type of processing used. Da Mota et al. [21] and Pereira et al. [22] used fermentation in closed bioreactors to favor aroma precursor compounds produced by the microbiota. Thus, these authors proved that even though yeasts as starter cultures present different behaviors in different coffee-producing regions and varieties (Bourbon Amarelo, Catucaí, and Rubi), the S. cerevisiae CCMA 0543 strain was able to increase the sensory profile of the beverage.
In this way, using the selected starter culture and the SIAF method intensified the exotic sensory profile of the coffee. However, different fermentation times can result in different microbial populations, chemical compositions, and, consequently, different sensory profiles in the beverage. Furthermore, the inoculation of S. cerevisiae CCMA0543 is expected to contribute to more pronounced exotic sensory notes at all fermentation times. Therefore, this work aimed to investigate the chemical, microbiological, and sensory changes in coffees submitted to different fermentation durations.
Materials and methods
Harvesting and processing the coffee
Ripe fruits of Coffea arabica cv Mundo Novo were harvested mechanically in the Condado farm, located in Santa Rita do Sapucaí (latitude: 22° 14’ 60’’ south, longitude: 45° 43’ 11’’ west) in the southwestern region of Minas Gerais, Brazil. After mechanized harvesting, the fruits were sent to a hydraulic separator to separate grains with different densities. Then, the coffee fruits that sank were manually placed on a raised bed to select the ripe fruits.
Each batch for fermentation, performed in 100-L high-density polyethylene bioreactors by the SIAF method [21, 22], included 75 kg of coffee fruit. Two treatments were performed: (1) Inoculated with Saccharomyces cerevisiae CCMA 0543 at the beginning of the fermentation process and (2) Spontaneous fermentation (without inoculation). The natural coffee was fermented for 0, 24, 48, 72, and 96 h. During the entire fermentation process, the coffee fruits obtained 51% of moisture. The coffees were peeled after each treatment reached the expected fermentation time (PENAGOS/DCV-183). Then, the coffees were spread on a suspended terrace for drying in the sun (beginning of drying) until reaching the desired water content (10.8 to 11.5% wet basis) (end of drying).
The yeast culture starter was grown in YEPG medium [g.L− 1: 10 yeast extract (HiMedia)], ten peptone (HiMedia)], 20 glucose (Dinamica), 20 agar (HiMedia)] at 30 °C and replicated every 24 h [16]. Yeast cells were recovered by centrifugation (7000 rpm, 10 min) and resuspended in 1 L of sterile peptone water [1 g.L− 1 peptone (Himedia)]. The solution was sprayed over the coffee fruit, reaching a concentration of approximately seven cells.g− 1 of coffee. Samples for chemical and microbiological analyses were stored at -20 °C. Fermentation was performed in triplicate.
Microbiological analysis
Microbial quantification and isolation
Microorganisms were isolated and quantified only in spontaneous fermentation samples, as the objective was to understand the microbiota naturally present in the coffee fruits used in this work. Thus, isolation was performed at the beginning (0 h), middle (48 h), and end of fermentation (96 h). Coffee samples (10 g) were homogenized in 90 ml of peptone water [0.1% v/w peptone bacteriology (Himedia)] in an orbital shaker (200 rpm − 20 min), and serial dilutions were performed. Mesophilic bacteria were enumerated in NA medium [Nutrient Agar (HiMedia)], and lactic acid bacteria were enumerated in MRS agar [De Man, Rogosa, and Sharpe (HiMedia)]. Both media were supplemented with 0.1% nystatin to inhibit filamentous fungi and yeast growth. The yeasts were enumerated in YEPG [g.L− 1: 10 yeast extract (HiMedia), ten peptone (HiMedia), 20 glucose (Dinamica), and 20 agar (HiMedia)]. Mesophilic and lactic acid bacteria were grown at 37 °C for 48 h. Yeast was grown at 28 °C for 48 h. After the incubation period, the number of colony-forming units (CFU) was obtained. Colonies were randomly picked in a number equal to the square root of the number of colonies on counted plates [23]. All samples of microbial analysis were performed in triplicate.
Identification by protein profile analysis (MALDI-TOF MS)
The isolated strains (228) were submitted to protein profile analysis in MALDI-TOF MS (Matrix-Assisted Laser Desorption Ionization - Time Of Flight Mass Spectrometer). As described, the cells were grown in plates using a specific culture medium for each taxonomic group and incubated for 18 h. Each colony (20 mg) was aseptically transferred to microtubes. Bacterial proteins were extracted with 6 µL organic solution [ethanol/acetonitrile/trifluoroacetic acid (10%), 1:1:1], and 6 µL of formic acid (25%) was used to yeast isolates. The microtubes were agitated in a vortex for 30 s and sonicated for 5 min. Aliquots of 0.6 µL of the resulting suspension were transferred to MALDI-TOF plates. Next, 1 µL of matrix solution [a-cyano-4-hydroxycinnamic acid (ACSH); 10 mg ACSH.mL− 1] was added. Escherichia coli K12 was employed as a standard for MALDI-TOF external calibration [24]. Each isolate was analyzed in triplicate to measure the quality and reproducibility of the spectra. The MALDI-TOF Microflex LT spectrometer (Bruker Daltonics, Bremen, Germany) analyzed the samples using the MALDI Biotyper 3.0 identification system. Strains exhibiting values below 1.7 were selected for DNA sequencing.
Molecular identification of bacteria and yeasts
DNA extraction from isolates was performed according to Cocolin et al. [25]. The bacterial community DNA was amplified with primers 27 F (5’AGAGTTGATCCTGCTCAG 3’) and 1512R (5’ACGGCTACCTTGTACGACT 3’) [26] covering the 16 S rRNA region. Yeast community DNA was amplified using ITS1 (5’-CGTAGGTGAACCTGCG-3’) primer and ITS4 (5’-TCCTGCTTATTGATGC-3’) [27]. Reactions were incubated in 25-µL volumes containing 12.5 µL of the top Taq Master Kit (Qiagen), 0.5 of each primer, three µL of DNA mold, and 8.5 µL of ultrapure water according to the manufacturer’s instructions.
The reactions were undertaken in a thermal cycler under the following conditions: 94 °C for 2 min, 30 cycles (94 °C for 40 s, 58 °C for 40 s, and 72 °C for 1 min and 30 s), and a final extension of 72 °C for 7 min. The amplicon quality was confirmed by 1.0% agarose gel electrophoresis. The amplified PCR products were purified using a kit (Qiagen) and sent for sequencing to GoGenetic (Curitiba, Brazil). The sequences were aligned using a BioEdit 7.7 sequence alignment editor and compared to the BLAST algorithm’s GenBank database (National Center for Biotechnology Information, Maryland, USA).
Behavior population of S. cerevisiae by real-time PCR (qPCR)
The total DNA of coffee samples was extracted during the fermentation in the bioreactor (0, 24, 48, 72, and 96 h) at the beginning and end of drying for both treatments: spontaneous and inoculated fermentation. DNA extraction was performed as described by Cocolin et al. [25]. Three grams of coffee were homogenized with 5 ml of ultrapure water for 10 min and centrifuged at 9000 rpm and 4 °C for 10 min. The resulting pellet was used for DNA extraction.
Specific primers for S. cerevisiae (SC-5fw 5’-AGGAGTGCGTTCTTGTAAAG-3’ and SC-3bw 5’-TGAAATGCGAGATTCCT-3’) used in this study were described previously by Díaz et al. [28]. The specificity of each primer pair was confirmed by searching GenBank using BLAST (http://www.ncbi.nlm.nih.gov/BLAST/). The qPCR was performed using the Rotor-Gene Q System (Qiagen, Hombrechtikon, ZH, Switzerland) according to Batista et al. [29] with modifications. Each reaction mixture consisted of 12.5 µl of 2× Mix Master Gene SYBR Green PCR (Qiagen, Stockach, Konstanz, Germany), 0.5 µM of each primer (Invitrogen, São Paulo, SP, Brazil), and one µl of DNA extracted from coffee beans for a total volume of 25 µl. The mixture was heated to 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 10 s and pairing/extension at 60 °C for 15 s. The cycle temperature was subsequently increased by one °C every 5 s from 50 °C to 99 °C to obtain the melting curve. All analyses were performed in triplicate. The DNA concentration was limited to 50 ng per analysis. The standard curve was generated from serial dilutions (108 to 104 cells.mL− 1) of DNA extracted from S. cerevisiae yeast. Each point on the calibration curve was measured in triplicate.
Chemical analysis
Carbohydrates and organic acids
The natural coffee was evaluated during fermentation in the bioreactor (0, 24, 48, 72, and 96 h) at the beginning and end of drying for both treatments: spontaneous and inoculated fermentation. Organic acids (malic, lactic, acetic, butyric, propionic, citric, oxalic, succinic, and tartaric) and carbohydrates (fructose, glucose, and sucrose) were analyzed.
Each sample (3 g) was homogenized in 20 mL of Milli-Q water per vortex at room temperature for 10 min. The samples were centrifuged at 8000 rpm at four °C for 10 min. The supernatant’s pH was adjusted to pH 2.11 using the perchloric acid solution and centrifuged under the same conditions. The supernatant was filtered through a 0.22-µm cellulose acetate filter and stored at -18 °C until analysis. The operational conditions were performed as Evangelista et al. [17] described.
Carbohydrates and organic acids were analyzed using a High-performance liquid chromatography (HPLC) system (Shimadzu). Carbohydrates were performed with an ion exclusion column (Shim-pack SCR-101 C, 7.9 mm x 30 cm) at a temperature of 50 °C with an RID detection system. Water was employed as the eluent at a flow rate of 0.6 mL.min− 1. The acids were prepared with a Shim-pack SCR-101 H (7.9 mm x 30 cm) temperature of 30 °C with a UV absorbance (210 nm) detection system. Compound quantification was performed using calibration curves generated using different concentrations of standard compounds [malic, propionic, and citric acids (Merck, Darmstadt, Germany); lactic, oxalic, and tartaric acids (Sigma Chemical, Saint Louis, MO, USA); acetic and succinic acids (Sigma Aldrich, Munich, Germany); butyric acid (Riedel-de Haen, Seelze, Germany)] and analyzed using the same conditions for the samples. The analysis was performed in duplicate.
Analysis of volatile compounds
Volatile compounds of roasted beans were identified using QP2010 gas chromatography coupled with mass spectrometry (GC-MS) (Shimadzu) using a 20 M (30 m × 0.25 mm × 0.25 mm) Carbo-Wax silica capillary column. The extraction was performed using solid-phase microextraction (HS-SPME), according to Evangelista et al. [17]. The roasted coffee (2 g) was macerated with liquid nitrogen and placed in a 15-mL vial, hermetically sealed. The samples were heated to 60 °C for 15 min. The fiber was placed at 60 °C and exposed for 30 min. The desorption time in the column was 2 min. The operational conditions were performed as described by Visintin et al. [30]. The mass spectra of each detected compound were compared with the NIST11 library, and a series of alkanes (C10 - C40) was employed to calculate the retention index (IR) [31].
Sensory analysis
The coffee samples of spontaneous and inoculated fermentation were used for sensory analysis. The samples were stored in hermetically sealed bags at 16 °C and were prepared according to the Specialty Coffee Association [32, 33]. According to the Green Coffee Grading Protocols, the grains were separated on the size 16 sieves above, and the grains with defects were removed (broken grains, parchment, and others). Then, the temperature versus roasting time curve was developed to standardize the roasting process for all samples. The beans’ total roasting time and final color were defined following the guidelines of Cupping Protocols. Thus, the total roasting time was set as 9 min, and the Agtron disc no. 60 (SCA Roast Color Classification System – Discs) was used to define the final color of the beans. Samples (100 g) were roasted in a Leogap-type TP2 roaster with a standardized initial roasting temperature of 170 °C and ground in an electric grinder, Copacabana model from Mahlkonig company. An 8.25 g of coffee per 150 mL of water was employed for coffee preparation. In addition, a panel of six trained coffee experts with Q-Grader coffee certificates evaluated the samples.
The dominance analysis of sensations over time (TDS) was used to determine the primary characteristics of fermented and spontaneous coffee. The most relevant coffee attributes, such as fruit, chocolate, caramel, spice, woody, chestnut, wine, and herbaceous flavors, were defined in a previous sensory analysis. Samples coded with three digits were submitted in a balanced and evaluated in three replicates. The analysis duration was 20 s for each sample, with an additional delay of 5 s for each sample to begin the sensory perception. During the 25 s, the judges indicated which attribute they were feeling. TDS curves were obtained according to the methodology proposed by Pineau et al. [34]. The software used was SensoMaker, version 1.8 [35]. The analysis was performed in triplicate.
Statistical analysis
The experiment was performed with a completely randomized design. A 2 × 5 factorial treatment arrangement was used to analyze the results of the S. cerevisiae population by qPCR, carbohydrates, and organic acids, with two treatments (spontaneous fermentation and inoculated with S. cerevisiae CCMA 0543) and five times of fermentation (0, 24, 48, 72, and 96 h). Microbial count, carbohydrates, and organic acid were evaluated according to the analysis of variance (ANOVA), and Scott Knott’s 5% probability level test was used to compare means. Statistical analyses were performed using Sisvar 5.6 software [36].
A heatmap was generated using the statistical software R to evaluate the differences between the bacterial and yeast community populations [37]. Microbial abundance was calculated based on the total population.
Results
Microbiological analysis
The microbial population was evaluated every 24 h during the spontaneous fermentation process of coffee fruit and in the drying stage. Mesophilic bacteria population during the coffee fruit fermentation (5.37 to 6.40 Log CFU.g− 1), lactic acid bacteria (4.90 to 6.08 Log CFU.g− 1), and yeasts (3.62 to 5.01 Log CFU.g− 1) increased significantly (p < 0.05) in the first 48 h of the fermentation process. After this period, there was a significant (p < 0.05) decline in the microbial population (Table 1). After removing part of the pericarp for drying, the bacteria population was reduced by up to 30% (Table 1). At the end of drying, the bacterial population was lower, and there was no significant difference at different times (< 2 Log CFU.g− 1). The intrinsic conditions of the seed favor the permanence of yeasts during drying, which was higher at the end of drying for 0 h (5.19 Log CFU.g− 1) and 48 h (5.47 Log CFU.g− 1) of fermentation.
Table 1.
Population of microorganisms presents at spontaneous fermentation, beginning, and end of drying process of coffee
| Microorganism (CFU.g-1) | Fermentation time (h) | ||||
|---|---|---|---|---|---|
| 0 | 24 | 48 | 72 | 96 | |
| Mesophilic Bacteria | |||||
| SIAF Fermentation | 5.37 b | 5.29 b | 6.40 a | 5.16 b | 5.07 b |
| Start of Drying | 3.67 b | 3.62 b | 4.88 a | 3.48 b | 4.92 a |
| End of Drying | < 2 a | < 2 a | < 2 a | < 2 a | < 2 a |
| Lactic Bacteria | |||||
| SIAF Fermentation | 4.90 c | 4.87 c | 6.08 a | 4.45 d | 5.19 b |
| Start of Drying | 3.89 b | 4.01 b | 4.98 a | 3.30 c | 3.52 c |
| End of Drying | < 2 a | < 2 a | < 2 a | < 2 a | < 2 a |
| Yeasts | |||||
| SIAF Fermentation | 3.62 b | 4.13 b | 5.01 a | 4.05 b | 3.95 b |
| Start of Drying | 4.00 b | 5.16 a | 3.09 c | 3.30 c | 3.87 b |
| End of Drying | 5.19 a | 3.28 c | 5.47 a | 4.26 b | 3.71 c |
The average of the populations in different times (0, 24, 48, 72, and 96 h) was analyzed for SIAF fermentation, beginning and end of drying, separately. Different lowercase letters (a-d) on lines indicate a statistically significant difference (p < 0.05) by the Scott - Knott’s test
Twenty species of mesophilic bacteria, four lactic acid bacteria, and 12 yeasts were identified (Supplementary material – Table S1) and classified according to abundance (Fig. 1). Microbacterium testaceum, Pantoea agglomerans, Pantoea sp, Cystofilobasidium ferigula, Dipodascaceae sp., Meyerozyma caribbica, Saccharomyces cerevisiae, and Staphylococcus saprophyticus were the most abundant species at the beginning of the fermentation process. After 48 h, Bacillus safensis, Leuconostoc mesenteroides, Gluconobacter cerinus, Pichia kluyveri, Saturnispora dispora, Torulaspora delbrueckii, Staphylococcus hominis, Staphylococcus pasteuri, and Staphylococcus warneri were determined to be most abundant. Acetobacter orientalis, Bacillus megaterium, Bacillus tequilensis, Candida glabrata, Issatchenkia orientalis, and Pichia kudriavzervi showed better growth at the end of the fermentation process.
Fig. 1.
Heatmap showing the abundance of microbial population found during spontaneous coffee fermentation (without inoculation). The yellow color indicates significant abundance, while the blue color indicates minor abundance
Behavior population of S. cerevisiae
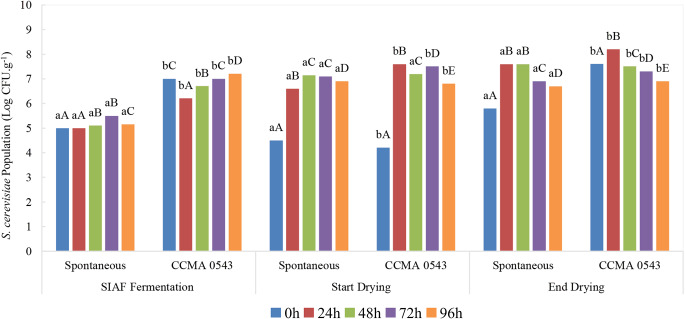
The dynamic behavior of the S. cerevisiae population during the spontaneous and inoculated fermentation processes was assessed by qPCR (Fig. 2). The population differed significantly (p < 0.05) during SIAF fermentation beginning and end of drying between treatments and at all fermentation times. The spontaneous fermentation exhibited lower density (5.0 to 5.1 Log CFU.g− 1) than the inoculated fermentation (7.0 to 7.2 Log CFU.g− 1), which was already expected. Inoculated treatment with S. cerevisiae showed the highest population at 96 h (7.2 CFU.g− 1) in SIAF fermentation.
Fig. 2.
Interactive effect of fermentation time and treatment (spontaneous and inoculated fermentation with S. cerevisiae CCMA 0543) on behavior of the population of S. cerevisiae yeast (Log CFU.g− 1). Spontaneous fermentation (without inoculum); CCMA 0543: inoculated fermentation with S. cerevisiae CCMA 0543. The population average in different treatments was analyzed for SIAF fermentation, start, and end of drying separately. Different lowercase letters (a-b) indicate a statistically significant difference (p < 0.05) by the Scott - Knott’s test evaluating the interaction in treatments. Different uppercase letters (A-E) indicate a statistically significant difference (p < 0.05) by the Scott - Knott’s test evaluating the interaction in time of fermentation
Even during drying, fermentation time also influenced the yeast population. The treatments differed significantly (p < 0.05) at the beginning of drying, except 48 h fermentation time (7.15 and 7.2 Log CFU.g− 1 for spontaneous and inoculated, respectively). A significant increase in the S. cerevisiae population was shown as a function of fermentation time. The first 24 h of fermentation showed a higher growth of S. cerevisiae. At the end of drying, the inoculated treatment showed the highest population (8.2 Log CFU.g− 1) within 24 h of fermentation.
Carbohydrates and organic acids
The consumption of sugars and organic acids was evaluated by HPLC (Tables 2 and 3). Reduced concentrations of sucrose were detected in the whole coffee fruit. Initial concentrations were significantly different (p < 0.05) for treatments (Table 2). No significant difference (p > 0.05) was detected in spontaneous fermentation until 72 h. However, inoculated fermentation decreased in the first 24 h of fermentation. Spontaneous and inoculated fermentation reduced 92% and 94% of the initial sucrose concentration in the first 24 h.
Table 2.
Interactive effect of fermentation time (0, 24, 48, 72, and 96 h) and treatment (spontaneous and inoculated fermentation with S. cerevisiae CCMA 0543) on carbohydrates concentration (mg.g-1) during the SIAF fermentation process, beginning and end of drying
| Carbohydrates (mg.g-1) | |||||||
|---|---|---|---|---|---|---|---|
| Fermentation time (h) | Sucrose | Glucose | Fructose | ||||
| Spont | Inoc | Spont | Inoc | Spont | Inoc | ||
| SIAF Fermentation | |||||||
| 0 | 0.12 aA | 2.58 bA | 25.69 aA | 37.50 bA | 44.30 aA | 58.33 bA | |
| 24 | 0.01 aA | 0.16 bB | 19.66 aB | 23.73 bB | 35.15 aB | 41.09 bB | |
| 48 | 0.04 aA | 0.04 aB | 17.55 aC | 2.05 bC | 27.44 aC | 3.49 bC | |
| 72 | 0.03 aA | 0.08 aB | 6.20 aD | 5.23 aD | 14.55 aD | 3.34 bC | |
| 96 | 0.62 aB | 0.08 bB | 2.40 aE | 2.24 aC | 2.82 aE | 2.26 aC | |
| Start of Drying | |||||||
| 0 | 3.31 aA | 3.05 bA | 9.94 aA | 10.43 bA | 16.08 aA | 16.14 aA | |
| 24 | 2.19 aA | 1.01 bB | 4.85 aB | 3.59 bB | 9.51 aB | 6.37 bB | |
| 48 | 2.01 aB | 1.03 bB | 3.88 aC | 3.58 aB | 7.26 aC | 1.79 bC | |
| 72 | 1.27 aC | 1.78 aB | 3.64 aD | 2.09 bC | 3.41 aD | 2.48 bC | |
| 96 | 1.48 aC | 0.44 bC | 3.19 aC | 0.61 bD | 4.38 aE | 1.97 bC | |
| End of Drying | |||||||
| 0 | 4.20 aA | 0.65 bA | 35.61 aA | 20.63 bA | 64.22 aA | 33.11 bA | |
| 24 | 1.98 aB | 0.23 bA | 17.76 aB | 1.63 bB | 47.33 aB | 5.43 bB | |
| 48 | 1.71 aB | 0.19 bA | 2.89 aC | 0.51 bC | 10.87 aC | 1.81 bC | |
| 72 | 0.21 aC | 0.15 aA | 1.34 aD | 0.02 bC | 6.75 aD | 1.09 bC | |
| 96 | - | 0.25 A | 0.08 E | - | 10.92 aC | 1.06 bC | |
Not detected. Spont: Spontaneous fermentation (without inoculum); Inoc: inoculated fermentation with S. cerevisiae CCMA 0543. The average concentration of each carbohydrate in different treatments was analyzed for SIAF fermentation, start, and end of drying separately. Different lowercase letters (a-b) on lines indicate a statistically significant difference (p < 0.05) by the Scott - Knott’s test evaluating the interaction of each carbohydrate in treatments. Different uppercase letters (A-E) on columns indicate a statistically significant difference (p < 0.05) by the Scott - Knott’s test evaluating the interaction of each carbohydrate in time of fermentation. Results are shown as the mean expressed on a dry weight basis
Table 3.
Interactive effect of fermentation time (0, 24, 48, 72, and 96 h) and treatment (spontaneous and inoculated fermentation with S. cerevisiae CCMA 0543) on organic acids concentration (mg.g-1) during the SIAF fermentation process, beginning and end of drying
| Acids (mg.g-1) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fermentation time (h) | Citric | Malic | Succinic | Latic | Acetic | Propionic | |||||||
| Spont | Inoc | Spont | Inoc | Spont | Inoc | Spont | Inoc | Spont | Inoc | Spont | Inoc | ||
| SIAF Fermentation | |||||||||||||
| 0 | 9.25 aA | 15.14 bA | 4.69 aA | 5.87 bA | 1.30 aA | 2.08 bA | 0.22 aA | 0.25 Aa | 8.49 aA | 12.87 bA | 0.02 A | - | |
| 24 | 13.35 aB | 2.43 bB | 3.99 aB | 3.76 bB | 1.59 aB | 2.75 bB | 0.92 aB | 2.18 bB | 12.93 aB | 15.71 bB | 0.45 aA | 0.29 bA | |
| 48 | 1.06 aC | 2.50 aB | 0.85 aC | 0.23 bC | 2.06 aC | 1.09 bC | 3.00 aC | 1.82 bC | 10.80 aC | 2.67 bC | 0.43 aB | 0.22 bA | |
| 72 | 3.88 aC | 10.24 bC | 0.26 aD | 0.26 aC | 1.70 aD | 3.10 bD | 2.28 aD | 3.14 bD | 4.30 aD | 1.90 bD | 0.37 aB | 0.29 aA | |
| 96 | 18.67 aD | 14.40 bA | 0.16 aE | 0.20 aC | 3.30 aE | 5.04 bE | 2.62 aE | 3.92 bE | 3.26 aE | 10.26 bE | 0.14 aA | 0.71 bB | |
| Start of Drying | |||||||||||||
| 0 | 4.17 aA | 4.56 aA | 1.35 aA | 1.54 bA | 0.97 aA | 0.93 aA | 0.11 aA | 0.12 aA | 4.08 aA | 4.25 bA | - | 0.05 A | |
| 24 | 1.26 aB | 2.58 bB | 0.45 aB | 0.33 bB | 0.51 aB | 0.46 bB | 0.27 aB | 0.29 aB | 2.93 aB | 2.26 bB | 0.02 A | - | |
| 48 | 0.82 aB | 1.75 bC | 0.20 aC | 0.12 bC | 0.69 aC | 0.55 bC | 0.61 aC | 0.53 bC | 2.91 aB | 0.68 bC | 0.07 A | - | |
| 72 | 3.26 aC | 4.92 bA | 0.18 aC | 0.26 bD | 0.83 aD | 1.21 bD | 0.24 aD | 0.40 bD | 0.89 aC | 1.37 bD | - | 0.57 B | |
| 96 | 3.21 aC | 2.62 aB | 0.18 aC | 0.18 aE | 0.83 aD | 0.77 bE | 0.44 aE | 0.40 bD | 0.79 aD | 0.63 bE | 0.01 aA | 0.03 aA | |
| End of Drying | |||||||||||||
| 0 | 9.03 aA | 14.35 bA | 4.12 aA | 4.08 aA | 10.37 aA | 5.82 bA | 0.19 aA | 0.31 bA | 10.52 aA | 11.79 bA | 0.30 aA | 0.39 aA | |
| 24 | 6.33 aB | 8.63 bB | 0.63 aB | 0.21 bB | 2.19 aB | 2.50 bB | 1.05 aB | 1.56 bB | 4.87 aB | 6.52 bB | 0.10 aA | 0.26 aA | |
| 48 | 3.96 aC | 2.15 bC | 0.18 aC | 0.20 aB | 2.71 aC | 3.86 bC | 1.59 aC | 2.37 bC | 3.84 aC | 2.89 bC | 0.16 aA | 0.19 aA | |
| 72 | 4.73 aD | 3.40 bD | 0.99 aD | 0.63 bC | 3.23 aD | 6.44 bD | 1.46 aD | 1.84 bD | 2.54 aD | 1.73 bD | - | 0.27 A | |
| 96 | 1.26 aE | 1.62 bE | 0.28 aC | 0.27 aB | 4.22 aE | 4.36 bE | 2.88 aE | 5.35 bE | 1.71 aE | 0.19 bE | 0.11 aA | 0.12 aA | |
Not detected. Spont: Spontaneous fermentation (without inoculum); Inoc: inoculated fermentation with S. cerevisiae CCMA 0543. The average concentration of each acid in different treatments was analyzed for SIAF fermentation, start, and end of drying separately. Different lowercase letters (a-b) on lines indicate a statistically significant difference (p < 0.05) by the Scott - Knott’s test evaluating the interaction of each acid in treatments. Different uppercase letters (A-E) on columns indicate a statistically significant difference (p < 0.05) by the Scott - Knott’s test evaluating the interaction of each acid in time of fermentation. Results are shown as the mean expressed on a dry weight basis
Glucose (spontaneous – from 25.69 to 2.40 mg.g− 1; inoculated – from 37.50 to 2.24 mg.g− 1) and fructose (spontaneous – from 44.30 to 2.82 mg.g− 1; inoculated – from 58.33 to 2.26 mg.g− 1) concentrations decreased significantly over time. There was no significant difference between treatments after 72 and 96 h fermentation (Table 2). The highest reduction percentage occurred after 96 h in spontaneous fermentation, reaching 91% concerning the initial glucose concentration. In inoculated fermentation, the reduction percentage increased gradually and reached 95% within 48 h of fermentation. Fructose concentration showed a faster reduction in the inoculated treatment, reaching 94% concerning the initial concentration with only 48 h, while spontaneous fermentation reached the same percentage at 96 h.
At the beginning of the drying process, sucrose concentrations differed significantly (p < 0.05) between treatments. The highest concentrations of glucose (9.94 mg.g− 1 – spontaneous; 10.43 mg.g− 1 - inoculated) and fructose (16.08 mg.g− 1 – spontaneous; 16.14 mg.g− 1 - inoculated) were obtained at 0 h of fermentation and decreased significantly over time (Table 2). Inoculated fermentation reduced 67% of the initial sucrose concentration in 24 h, while spontaneous fermentation reduced 62% in 72 h. After 96 h of fermentation, glucose was reduced by 68% and 94% in spontaneous and inoculated fermentation, respectively. The higher percentage of fructose reduction was 89% at 48 h for inoculated fermentation, while spontaneous fermentation reduced by 79% at 72 h.
The same behavior was observed at the end of drying. Spontaneous fermentation was reduced until 100% of the initial sucrose concentration was reached in 96 h. However, inoculated fermentation decreased by 77% within 72 h. After 24 h of fermentation, glucose was reduced by 50% in spontaneous fermentation, while inoculated fermentation reached 92% simultaneously. The higher percentage of fructose reduction was 95% at 48 h for inoculated fermentation, while spontaneous fermentation reduced only 89% at 72 h.
Citric, acetic, and malic acids were detected in higher concentrations at the beginning of the fermentation process (0 h) (Table 3). Succinic and lactic acid concentrations increased during inoculated and spontaneous fermentation, with increases of up to 94%. Higher propionic acid concentrations were found in coffee inoculated with 72 h of fermentation (0.71 mg.g− 1). Butyric and tartaric acid were not detected.
At the beginning of drying, there were significant differences (p < 0.05) in the organic acids concentrations obtained between inoculated and spontaneous fermentations (Table 3). Similar results were found at the end of drying. Fermentation times and treatments influenced the concentrations of acetic, citric, lactic, and succinic acids (Table 3). The malic acid did not differ significantly after 24, 48, and 96 h of the inoculated fermentation. The same behavior was observed after 48 and 96 h of spontaneous fermentation.
Volatile compounds
One hundred twenty-one volatile compounds were detected in roasted coffee beans (Supplementary material – Table S2). Of these compounds, 22 were found only in coffees inoculated with S. cerevisiae CCMA0543, and 17 were found only in coffees with spontaneous fermentation. These compounds were grouped into 14 chemical classes: acids (10), alcohols (12), aldehydes (7), esters (13), furans (17), ketones (14), lactones (2), pyrazines (16), pyridines (7), pyrroles (9), sulfur compounds (4), phenols (4), terpenes (3) and other compounds (4). 2-Furanmethanol and acetic acid are the main compounds detected during fermentation except for spontaneous fermentation in which acetic acid was abundant at 24 and 48 h. The PCA was performed to evaluate the correlations between chemical classes and fermentation times since similar samples tend to be grouped (Fig. 3).
Fig. 3.

Principal component analysis (PCA) of the volatile compounds classes identified by HS-SPME GC-MS in roasted beans produced after 0, 24, 48, 72, and 96 h of (a) spontaneous fermentation and (b) fermentation inoculated with S. cerevisiae CCMA0543. Spont: spontaneous fermentation; Inoc: fermentation inoculated with S. cerevisiae CCMA0543
In coffees with spontaneous fermentation, more significant correlations could be observed between 24 and 96 h fermentations (Fig. 3A). Ketone, methyl 6-methyl-2-pyridyl was only detected at these times and may contribute buttery and caramel sensory attributes. Coffee fermented for 96 h obtained more significant correlations with furans and pyridines. Among the compounds, 2-furan carboxaldehyde, 5-methyl, 2-furan methanol, 2-furan methanol, acetate, and furfuryl formate were highlighted (Supplementary material – Table S2).
Coffees fermented at 0, 48, and 72 h were not correlated with other fermentation times (Fig. 3A). Coffee with 0 h of fermentation correlated with pyrroles. Coffee fermented for 48 h is associated with acids, such as acetic acid. Finally, coffee fermented for 72 h was correlated with esters, aldehydes, and phenols. Among the compounds, pentanal and 2-methoxy-4-vinylphenol were more significant (Supplementary material – Table S2).
On the other hand, coffees fermented with S. cerevisiae CCMA0543 showed different results (Fig. 3B). More significant correlations were observed between the 72- and 96-hour fermentation. These fermentations correlated with phenols and furans, mainly 2-methoxy-4-vinylphenol, 2-furan carboxaldehyde, 5-methyl-, 2-furan methanol, benzofuran, 2,3-dihydro- and furfural (Supplementary material – Table S2).
Furthermore, the 0 h and 48 h fermentations also showed correlations (Fig. 3B). However, coffee with 0 h fermentation showed more significant correlations with aldehydes, alcohols, ketones, and acids. The compounds 2-indecent, E-, 2,3-butanediol, and 2-propanone, 1-hydroxy- were found only then. On the other hand, 48 h fermented coffee showed a more significant correlation with esters, pyrroles, and lactones. Acetic acid, heptyl ester, benzoic acid, and 2-hydroxy-and ethyl ester were found only in this treatment (Supplementary material – Table S2).
Finally, coffees fermented for 24 h showed higher correlations with pyrazines (Fig. 3B). Pyrazine, 2,5-dimethyl-, pyrazine, 2-ethyl-3,5-dimethyl-, pyrazine, 2-ethyl-3-methyl- and pyrazine, 2-ethyl-6-methyl- were only detected at this fermentation time (Supplementary material – Table S2).
Sensory analysis
The sensory profile of coffee products was evaluated by temporal dominance sensation (TDS) (Fig. 4). The attributes that characterized spontaneously fermented and inoculated coffees were spice and caramel (0 h); wine, caramel, and wood (24 h); wood, chestnut, and caramel (48 h); caramel, chocolate, wine, and fruit (72 h); and chocolate, spices, wine, fruit, and herbaceous flavor (96 h). The coffees had different sensorial profiles, and the permanence of the sensation was more prolonged in the inoculated coffees with less overlapping of attributes. Fruit (72 and 96 h) and herbaceous flavor (96 h) attributes were dominant only in inoculated coffees.
Fig. 4.

Temporal Dominance Sensory of coffee beverage produced after 0, 24, 48, 72, and 96 h of spontaneous (a) and inoculated (b) fermentations
The dominant sensory attribute in spontaneously fermented coffee after 0 h was spice (5 to 11 s); after 24 h, it was winey. On the other hand, after 48 h, it was wood (6 to 8 s) and chestnuts (14 to 15 s); after 72 h, it was caramel (6 to 8 s), chocolate (8 to 10 s) and winey (15 to 6 s) and after 96 h it was chocolate (3 to 7 s), spices (11 to 15 s) and winey ( 16 to 17 s).
Regarding the coffees inoculated with S. cerevisiae, the predominant attributes after 0 h of fermentation were caramel (8 s to 10 s) and spices (10 s to 15 s). After 24 h, it was caramel (5 to 12 s) and woody (16 to 19 s), and after 48 h, it was caramel (6 to 8 s), woody (11 to 15 s) and chestnut (13 to 16 s). Finally, after 72 h, it was fruity (7 to 12 s), and after 96 h, it was chocolate (4 to 7 s), fruity (8 to 10 s), herbaceous (10 s until the end of the analysis) and winey (11 to 14 s ).
Discussion
The chemical and microbiological changes occurring in the spontaneously fermented and inoculated coffee were monitored during fermentation and drying. Yeast, lactic acid, and mesophilic bacteria were the primary microbial groups investigated during coffee fermentation.
Bacteria were detected at higher concentrations than other microbial groups in the first 72 h fermentation process. L. mesenteroides, L. brevis, and L. plantarum are lactic acid bacteria commonly observed during coffee fermentation [16, 17, 38]. After 48 h of fermentation, the bacterial metabolic activity showed high esters and lactic acid concentrations in both coffees. Organic acids reduce the medium’s pH, inhibiting undesirable microorganisms, such as ochratoxigenic fungi [39, 40].
The coffee fruit’s physical and nutritional changes during processing contribute to a reduction in bacterial population, favoring yeast development [41]. Ribeiro et al. [18] also evaluated the yeast S. cerevisiae CCMA 0543 in the Mundo Novo variety. However, the authors used semi-dry aerobic processing, and the yeast population at the end of drying was lower (approximately 4.3 Log CFU.g-1) than the SIAF method fermentation process (average 6.92 Log CFU.g− 1). Thus, this demonstrates that the SIAF fermentation method positively influences yeast survival.
The number of yeast cells may vary according to the microorganism and fermentation time [39]. The high yeast population detected after 48 (spontaneous) and 72 (inoculated) hours of fermentation can be explained by their ability to ferment the free sugars present in the mucilage in semi-aerobic or anaerobic conditions [12]. The reduction in microbial concentration was associated with the fruit’s intrinsic conditions [39].
S. cerevisiae and T. delbrueckii are commonly found during the fermentation process of coffee. In addition, these species are helpful starter cultures due to their excellent proteolytic activity, and they contribute positively to volatile compound profiles [15–17, 42]. Methyl hexadecanoate and acetic acid, heptyl ester are metabolites produced by S. cerevisiae and can contribute to the flavor’s balsam, creamy, fruity, mild, milky; and apricot, fresh, fruit, pear, rum, and woody, respectively [43, 44].
The intrinsic conditions, such as the beans’ low moisture, the remaining mucilage, and the strong adsorption forces between water and other constituents, such as nitrogenous substances, sugars, and chlorogenic acids, kept the yeast population viable until the end of the drying process [45].
Microbial activity influences the concentration of volatile and nonvolatile compounds in coffee. For example, lactic acid production occurred more rapidly in inoculated fermentation than in spontaneous fermentation. Acidity is an essential attribute of coffee quality, and its perception is related to the concentration of organic acids. The reduction in the concentration of malic acid was correlated with an increase in lactic acid concentration. This conversion occurs through the enzyme malate carboxy-lyase, which converts malic acid into lactic acid and CO2. Leuconostoc mesenteroides and Lactiplantibacillus plantarum can perform this conversion, also known as malolactic fermentation [46, 47].
Citric acid is an essential intermediate compound in plant metabolic life [48]. In coffee, the total acid content and the development of perceived acidity are crucial. After 96 h of fermentation, the two-fold increase in citric acid and succinic acid concentration may be associated with microbial metabolism and produced from both the citric acid and the glyoxylate cycles. The abundance of the genus Bacillus at the end of the fermentation process can be associated with an increase in population. In addition to this metabolic route, succinic acid can be produced by 4-aminobutanoate degradation and glutamate metabolism by mesophilic bacteria and yeasts [14, 49].
Propionic acid is a metabolite related to beverage quality, and low (1 mg.mL− 1) concentrations are enough to contribute positively to the sensory profile [50]. The lack of monitoring of the fermentation can create favorable conditions for the development of the bacteria Clostridium butyricum and its enzymes, which react with the chemical components of mucilage, producing the undesirable flavor of butyric acid in coffee [16, 17]. Várady [51] found that the anaerobic condition stimulates or inhibits the production of some volatiles, such as acetic acid and 2,3-butanediol, respectively. Volatile 2,3-butanediol is related to potentially defective coffee beans and overfermentation [52]. A bioreactor for coffee fermentation has become an essential tool for developing desirable aromatic compounds. For example, 3,5-dihydroxy-6-methyl-2,3-dihydropyran-4-one has been associated with coffees that have gone through the induced fermentation stage [53].
Ribeiro [19] evaluated coffee processed by the semi-dry or pulped method through aerobic fermentation with yeast starters and found the sensation of bitterness, characterized mainly by astringency and bitterness. Conversely, with the SIAF method, it was observed that the attributes, the dominance rate, and the sensation duration differentiated the spontaneously fermented and inoculated coffees. This result could be observed because TDS allows multiple sensory attributes to be recorded simultaneously over time, making it an excellent alternative for evaluating and differentiating complex products such as coffee [34]. Thus, the choice of type (with or without inoculation) and fermentation time (0, 24, 48, 72, and 96 h) will depend on the sensorial profile the producer intends to obtain. For example, in spontaneous fermentation, coffee fermented for 48 h was the only one that showed dominance of woody notes, and coffee fermented for 72 h was the only one that showed dominance of chestnuts. However, in the inoculated coffee, 72 h of fermentation showed high fruity dominance, and 96 h of fermentation was the only one with herbaceous notes. In addition, the reduced overlap of the coffee attributes provides a clearer perception of the flavor [54].
Da Silva Vale et al. [55] also observed that extended fermentation processes (48 and 72 h) allowed higher production of key metabolites and higher diversification of the sensory profiles of coffee beverages compared to 24 h of fermentation. Therefore, the SIAF method and S. cerevisiae inoculation generated specific flavors in the coffee beverage that can be selected from different fermentation times. This improved coffee quality and allowed producers to produce a quality beverage with unique sensory profiles. The operational cost of this process is offset by the higher value that specialty coffees can achieve [56].
Conclusion
Leuconostoc mesenteroides, Lactiplantibacillus plantarum, Staphylococcus warneri, Bacillus sp., and the yeasts Torulaspora delbrueckii, Hanseniaspora uvarum, and Meyerozyma caribbica were the most abundant species detected in the Mundo Novo variety in the spontaneous fermentation and the different fermentation times influenced the microbiological, chemical, and sensory profiles of spontaneously fermented coffees and those inoculated with S. cerevisiae CCMA0543. Coffee processed using the SIAF method must remain in the bioreactor for at least 48 h and can enhance caramel notes. S. cerevisiae showed higher metabolic activity after 72 h of fermentation, increasing the dominance of fruity notes. Different sensory profiles of the beverage can be obtained by the type of fermentation (with and without inoculation) and by the time (0, 24, 48, 72, and 96 h) that the coffee remains under induced anaerobiosis. This diversity of flavors expands the consumer market, allowing producers to adapt their coffee to meet their target audience’s preferences better.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the following Brazilian agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). We also thank Syngenta (NUCOFFEE) for supporting this work.
Author contributions
All authors contributed to the study’s conception and design. The first draft of the manuscript was written, reviewed, and edited by J.M.C.P., who was also responsible for material preparation, methodology development, data collection, and formal data evaluation. L.G.A.S. was responsible for material preparation, methodology development, and data collection. N.N.B., L.S.R., and P.M.M.M. made critical, specific comments, reviewed the presentation of the data, and edited the manuscript. F.M.B. was also responsible for methodology development, data collection, and providing conditions for the experiments. D.R.D. contributed substantially to the conception or design of the work and interpretation of data. R.F.S. supervised J.P. during the whole experiment period and also performed the original writing of the manuscript, supervision, and project administration.
Funding
This work was supported by the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Data availability
Data will be made available at a reasonable request.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Campo E, Ballester J, Langlois J, Dacremont C, Valentin D (2010) Comparison of conventional descriptive analysis and a citation frequency-based descriptive method for odor profiling: an application to Burgundy Pinot noir wines. Food Qual Prefer 21(1):44–55. 10.1016/j.foodqual.2009.08.001 [Google Scholar]
- 2.Contreras-Calderón J, Mejía-Díaz D, Martínez-Castaño M et al (2016) Evaluation of antioxidant capacity in coffees marketed in Colombia: relationship with the extent of non-enzymatic browning. Food Chem 209:162–170. 10.1016/j.foodchem.2016.04.038 [DOI] [PubMed] [Google Scholar]
- 3.Kwak HS, Jeong Y, Kim M (2018) Effect of yeast fermentation of Green Coffee beans on antioxidant activity and consumer acceptability. J Food Qual 2018:1–8. 10.1155/2018/5967130 [Google Scholar]
- 4.Martinez SJ, Bressani APP, Miguel MGdaCP, Dias DR, Schwan RF (2017) Different inoculation methods for semi-dry processed coffee using yeasts as starter cultures. Food Res Int 102:333–340. 10.1016/j.foodres.2017.09.096 [DOI] [PubMed] [Google Scholar]
- 5.CONAB CN de A. Boletim da Safra de Café. Published 2023. Accessed May 4 (2023) https://www.conab.gov.br/info-agro/safras/cafe
- 6.Braga AVU, Miranda MA, Aoyama H, Schmidt FL (2023) Study on coffee quality improvement by self-induced anaerobic fermentation: microbial diversity and enzymatic activity. Food Res Int 165:112528. 10.1016/j.foodres.2023.112528 [DOI] [PubMed] [Google Scholar]
- 7.Jimenez EJM, Martins PMM, Vilela AL, de O et al (2023) Influence of anaerobic fermentation and yeast inoculation on coffee’s viability, chemical composition, and quality. Food Biosci 51:102218. 10.1016/j.fbio.2022.102218 [Google Scholar]
- 8.Cassimiro DM, de Batista J, Fonseca NN HC, et al (2023) Wet fermentation of Coffea canephora by lactic acid bacteria and yeasts using the self-induced anaerobic fermentation (SIAF) method enhances the coffee quality. Food Microbiol 110:104161. 10.1016/j.fm.2022.104161 [DOI] [PubMed] [Google Scholar]
- 9.Lee LW, Cheong MW, Curran P, Yu B, Liu SQ (2016) Modulation of coffee aroma via the fermentation of green coffee beans with Rhizopus Oligosporus: I. Green coffee. Food Chem 211:916–924. 10.1016/j.foodchem.2016.05.076 [DOI] [PubMed] [Google Scholar]
- 10.Lee LW, Cheong MW, Curran P, Yu B, Liu SQ (2015) Coffee fermentation and flavor – an intricate and delicate relationship. Food Chem 185:182–191. 10.1016/j.foodchem.2015.03.124 [DOI] [PubMed] [Google Scholar]
- 11.Lee LW, Tay GY, Cheong MW, Curran P, Yu B, Liu SQ (2017) Modulation of the volatile and non-volatile profiles of coffee fermented with Yarrowia Lipolytica: I. Green coffee. LWT 77:225–232. 10.1016/j.lwt.2016.11.047 [Google Scholar]
- 12.Silva CF (2015) Microbial activity during coffee fermentation. Cocoa and Coffee Fermentation. CRC Taylor & Francis, Boca Raton, FL, pp 368–423 [Google Scholar]
- 13.Lian J, Mishra S, Zhao H (2018) Recent advances in metabolic engineering of Saccharomyces cerevisiae: New tools and their applications. Metab Eng 50:85–108. 10.1016/j.ymben.2018.04.011 [DOI] [PubMed] [Google Scholar]
- 14.Silva CF, Vilela DM, de Souza Cordeiro C, Duarte WF, Dias DR, Schwan RF (2013) Evaluation of a potential starter culture for enhance the quality of coffee fermentation. World J Microbiol Biotechnol 29(2):235–247. 10.1007/s11274-012-1175-2 [DOI] [PubMed] [Google Scholar]
- 15.Vilela DM, Pereira GV, de Silva M, Batista CF, Schwan LR RF (2010) Molecular ecology and polyphasic characterization of the microbiota associated with semi-dry processed coffee (Coffea arabica L). Food Microbiol 27(8):1128–1135. 10.1016/j.fm.2010.07.024 [DOI] [PubMed] [Google Scholar]
- 16.Evangelista SR, da Cruz Pedrozo Miguel MG, de Souza Cordeiro C, Silva CF, Marques Pinheiro AC, Schwan RF (2014) Inoculation of starter cultures in a semi-dry coffee (Coffea arabica) fermentation process. Food Microbiol 44:87–95. 10.1016/j.fm.2014.05.013 [DOI] [PubMed] [Google Scholar]
- 17.Evangelista SR, Silva CF, Miguel MGP da C, et al (2014) Improvement of coffee beverage quality by using selected yeasts strains during the fermentation in dry process. Food Res Int 61:183–195. 10.1016/j.foodres.2013.11.033 [Google Scholar]
- 18.Ribeiro LS, Ribeiro DE, Evangelista SR et al (2017) Controlled fermentation of semi-dry coffee (Coffea arabica) using starter cultures: a sensory perspective. LWT - Food Sci Technol 82:32–38. 10.1016/j.lwt.2017.04.008 [Google Scholar]
- 19.Ribeiro LS, Miguel MG da CP, Evangelista SR et al (2017) Behavior of yeast inoculated during semi-dry coffee fermentation and the effect on chemical and sensorial properties of the final beverage. Food Res Int 92:26–32. 10.1016/j.foodres.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 20.Bressani APP, Martinez SJ, Evangelista SR, Dias DR, Schwan RF (2018) Characteristics of fermented coffee inoculated with yeast starter cultures using different inoculation methods. LWT 2:212–219. 10.1016/j.lwt.2018.02.029 [Google Scholar]
- 21.Da Mota MCB, Batista NN, Rabelo MHS, Ribeiro DE, Borém FM, Schwan RF (2020) Influence of fermentation conditions on the sensorial quality of coffee inoculated with yeast. Food Res Int 136:109482. 10.1016/j.foodres.2020.109482 [DOI] [PubMed] [Google Scholar]
- 22.Pereira TS, Batista NN, Santos Pimenta LP et al (2022) Self-induced anaerobiosis coffee fermentation: impact on microbial communities, chemical composition and sensory quality of coffee. Food Microbiol 103:103962. 10.1016/j.fm.2021.103962 [DOI] [PubMed] [Google Scholar]
- 23.Sengun IY, Nielsen DS, Karapinar M, Jakobsen M (2009) Identification of lactic acid bacteria isolated from Tarhana, a traditional Turkish fermented food. Int J Food Microbiol 135(2):105–111. 10.1016/j.ijfoodmicro.2009.07.033 [DOI] [PubMed] [Google Scholar]
- 24.Lima-Neto R, Santos C, Lima N, Sampaio P, Pais C, Neves RP (2014) Application of MALDI-TOF MS for requalification of a Candida clinical isolates culture collection. Brazilian J Microbiol 45(2):515–522. 10.1590/S1517-83822014005000044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cocolin L, Bisson LF, Mills DA (2000) Direct profiling of the yeast dynamics in wine fermentations. FEMS Microbiol Lett 189(1):81–87. 10.1111/j.1574-6968.2000.tb09210.x [DOI] [PubMed] [Google Scholar]
- 26.Devereux R, Willis SG (1995) Amplification of ribosomal RNA sequences. In: Molecular Microbial Ecology Manual. Springer Neth 277–287. 10.1007/978-94-011-0351-0_19
- 27.Esteve-Zarzoso B, Belloch C, Uruburu F, Querol A (1999) Identification of yeasts by RFLP analysis of the 5.8S rRNA gene and the two ribosomal internal transcribed spacers. Int J Syst Evol Microbiol 49(1):329–337. 10.1099/00207713-49-1-329 [DOI] [PubMed] [Google Scholar]
- 28.Díaz C, Molina AM, Nähring J, Fischer R (2013) Characterization and dynamic behavior of wild yeast during spontaneous wine fermentation in steel tanks and amphorae. Biomed Res Int 2013:1–13. 10.1155/2013/540465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batista NN, Ramos CL, Ribeiro DD, Pinheiro ACM, Schwan RF (2015) Dynamic behavior of Saccharomyces cerevisiae, Pichia kluyveri and Hanseniaspora uvarum during spontaneous and inoculated cocoa fermentations and their effect on sensory characteristics of chocolate. LWT - Food Sci Technol 63(1):221–227. 10.1016/j.lwt.2015.03.051
- 30.Visintin S, Ramos L, Batista N, Dolci P, Schwan F, Cocolin L (2017) Impact of Saccharomyces cerevisiae and Torulaspora Delbrueckii starter cultures on cocoa beans fermentation. Int J Food Microbiol 257:31–40. 10.1016/j.ijfoodmicro.2017.06.004 [DOI] [PubMed] [Google Scholar]
- 31.Piccino S, Boulanger R, Descroix F, Shum Cheong Sing A (2014) Aromatic composition and potent odorants of the specialty coffee brew Bourbon Pointu correlated to its three trade classifications. Food Res Int 61:264–271. 10.1016/j.foodres.2013.07.034 [Google Scholar]
- 32.SCA SCA of A. Cupping Protocols (2018) Accessed May 10, 2020. https://sca.coffee/research/protocols-best-practices
- 33.Lingle TR (2011) The Coffee Cupper’s handbook: a systematic guide to the sensory evaluation of Coffee’s flavor. Specialty Coffee Association of America
- 34.Pineau N, Schlich P, Cordelle S et al (2009) Temporal dominance of sensations: construction of the TDS curves and comparison with time–intensity. Food Qual Prefer 20(6):450–455. 10.1016/j.foodqual.2009.04.005 [Google Scholar]
- 35.Pinheiro ACM, Nunes CA, Vietoris V (2013) SensoMaker: a tool for sensorial characterization of food products. Ciência E Agrotecnol 37(3):199–201. 10.1590/S1413-70542013000300001 [Google Scholar]
- 36.Ferreira DF (2014) Sisvar: a guide for its bootstrap procedures in multiple comparisons. Ciência E Agrotecnol 38(2):109–112. 10.1590/S1413-70542014000200001 [Google Scholar]
- 37.Team RC (2020) A language and environment for statistical computing. R Foundation for Statistical Computing. Published online 2020. Accessed January 9. https://www.r-project.org/
- 38.Ribeiro LS, Evangelista SR, da Cruz Pedrozo Miguel MG, van Mullem J, Silva CF, Schwan RF (2018) Microbiological and chemical-sensory characteristics of three coffee varieties processed by wet fermentation. Ann Microbiol 68(10):705–716. 10.1007/s13213-018-1377-4 [Google Scholar]
- 39.Haile M, Kang WH (2019) The role of microbes in Coffee Fermentation and their impact on Coffee Quality. J Food Qual 2019:1–6. 10.1155/2019/4836709 [Google Scholar]
- 40.Pereira GV, de Carvalho Neto M, Medeiros DP ABP, et al (2016) Potential of lactic acid bacteria to improve the fermentation and quality of coffee during on-farm processing. Int J Food Sci Technol 51(7):1689–1695. 10.1111/ijfs.13142 [Google Scholar]
- 41.Brando CHJ, Brando MFP (2015) Methods of coffee fermentation and drying. Cocoa and Coffee Fermentation. CRC Taylor & Francis Group, pp 341–365
- 42.Silva CF, Schwan RF, Sousa Dias Ë, Wheals AE (2000) Microbial diversity during maturation and natural processing of coffee cherries of Coffea arabica in Brazil. Int J Food Microbiol 60(2–3):251–260. 10.1016/S0168-1605(00)00315-9 [DOI] [PubMed] [Google Scholar]
- 43.Bardi L, Cocito C, Marzona M (1999) Saccharomyces cerevisiae cell fatty acid composition and release during fermentation without aeration and in absence of exogenous lipids. Int J Food Microbiol 47(1–2):133–140. 10.1016/S0168-1605(98)00203-7 [DOI] [PubMed] [Google Scholar]
- 44.Verstrepen KJ, Van Laere SDM, Vanderhaegen BMP et al (2003) Expression levels of the yeast alcohol acetyltransferase genes ATF1, Lg-ATF1, and ATF2 control the formation of a broad range of Volatile Esters. Appl Environ Microbiol 69(9):5228–5237. 10.1128/AEM.69.9.5228-5237.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martins PMM, Batista NN, Miguel MGdaCP, Simão JBP, Soares JR, Schwan RF (2020) Coffee growing altitude influences the microbiota, chemical compounds and the quality of fermented coffees. Food Res Int 129:108872. 10.1016/j.foodres.2019.108872 [DOI] [PubMed] [Google Scholar]
- 46.Koduru L, Kim Y, Bang J, Lakshmanan M, Han NS, Lee D-Y (2017) Genome-scale modeling and transcriptome analysis of Leuconostoc mesenteroides unravel the redox governed metabolic states in obligate heterofermentative lactic acid bacteria. Sci Rep 7(1):15721. 10.1038/s41598-017-16026-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krieger-Weber S, Heras JM, Suarez C (2020) Lactobacillus plantarum, a New Biological Tool to control malolactic fermentation: a review and an Outlook. Beverages 6(2):23. 10.3390/beverages6020023 [Google Scholar]
- 48.Pott DM, Osorio S, Vallarino JG (2019) From Central to Specialized Metabolism: an overview of some secondary Compounds Derived from the primary metabolism for their role in conferring nutritional and organoleptic characteristics to Fruit. Front Plant Sci 10. 10.3389/fpls.2019.00835 [DOI] [PMC free article] [PubMed]
- 49.Swiegers JH, Bartowsky EJ, Henschke PA, Pretorius IS (2005) Yeast and bacterial modulation of wine aroma and flavour. Aust J Grape Wine Res 11(2):139–173. 10.1111/j.1755-0238.2005.tb00285.x [Google Scholar]
- 50.Bertrand B, Boulanger R, Dussert S et al (2012) Climatic factors directly impact the volatile organic compound fingerprint in green Arabica coffee bean as well as coffee beverage quality. Food Chem 135(4):2575–2583. 10.1016/j.foodchem.2012.06.060 [DOI] [PubMed] [Google Scholar]
- 51.Várady M, Tauchen J, Fraňková A et al (2022) Effect of method of processing specialty coffee beans (natural, washed, honey, fermentation, maceration) on bioactive and volatile compounds. LWT 172:14245. 10.1016/j.lwt.2022.114245 [Google Scholar]
- 52.Toci AT, Farah A (2008) Volatile compounds as potential defective coffee beans’ markers. Food Chem 108:1133–1141. 10.1016/j.foodchem.2007.11.064 [DOI] [PubMed] [Google Scholar]
- 53.Li J, Wang S, Liu H-Y, Zhou H, Fu Y (2017) Effective hydrodeoxygenation of Stearic Acid and Cyperus Esculentus Oil into Liquid Alkanes over Nitrogen-Modified Carbon Nanotube-supported ruthenium catalysts. ChemistrySelect 2(1):33–41. 10.1002/slct.201601658 [Google Scholar]
- 54.Pineau N, Schilch P (2015) Temporal dominance of sensations (TDS) as a sensory profiling technique. Rapid Sens Profiling Techniques Elsevier 269–306. 10.1533/9781782422587.2.269
- 55.da Silva Vale A, Balla G, Rodrigues LRS, de Carvalho Neto DP et al (2022) Understanding the effects of Self-Induced Anaerobic Fermentation on Coffee beans Quality: Microbiological, metabolic, and sensory studies. Foods 12(1):37. 10.3390/foods12010037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Magalhães Júnior AI, de Carvalho Neto DP, de Melo Pereira GV, da Silva Vale A, Medina JDC, de Carvalho JC, Soccol CR (2021) A critical techno-economic analysis of coffee processing utilizing a modern fermentation system: implications for specialty coffee production. Food Bioprod Process 125:14–21. 10.1016/j.fbp.2020.10.010 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available at a reasonable request.