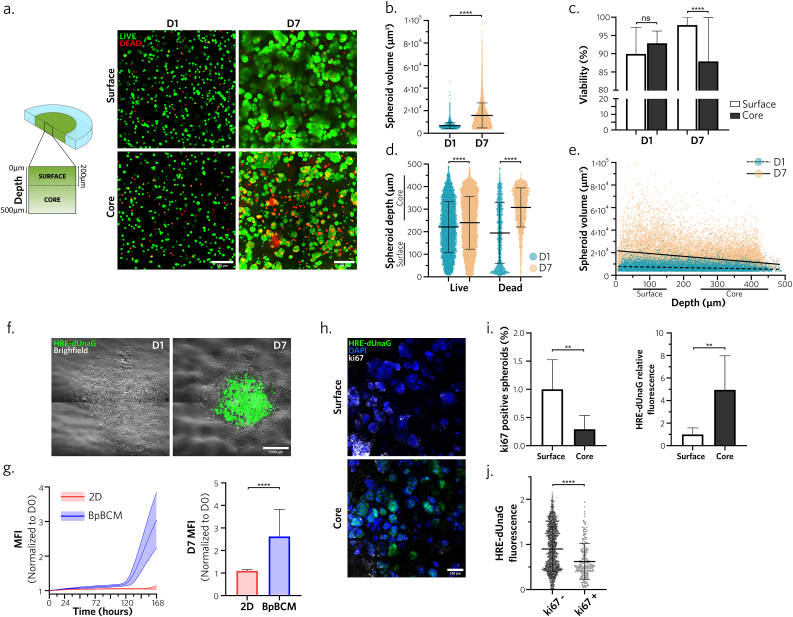
Fig. 2.
BpBCM cancer center develops a necrotic-like hypoxic core with a proliferative surface
a. BpBCM printed with MCF-7 cells in the cancer bioink and an empty ink for the periphery were incubated in Calcein-AM for live cells staining (green) and EthD-1 for dead cells staining (red) on day 1 and day 7. Shown are representative confocal scanning layer in the surface (<200 μm from medium contact) and core (>200 μm from medium contact) areas from 3D confocal acquisitions. Scale bars: 100 μm. b. Live spheroid volume quantifications on day 1 and 7 (N = 3, n = 18). c. Percent of viable cells in the model, in the surface and the core on day 1 and 7 (N = 3, n = 18). d. Live and dead cells depths in the model on day 1 and 7. e. Linear regression between live spheroid volume and depth on day 1(slope = −5.254) and day 7 (slope = −25.10). f. Timelapse imaging of BpBCM printed with MCF-7 transduced with HRE-dUnaG hypoxia reporter (green) merged with brightfield image on day 1 and day 7. Scale bar: 1000 μm. g. Quantification of HRE-dUnaG reporter mean fluorescence intensity over time (left) and at day 7 (right), in 2D MCF-7 HRE-dUnaG cells (red) and printed in the center of BpBCM (blue) (N = 3, n = 15–24). h. Immunofluorescence for ki67 (gray). BpBCM models printed with MCF-7 HRE-dUnaG reporter (green), nuclei stained with DAPI (blue). Scale bar: 100 μm. j. Spheroid positivity to ki67 (left) and HRE-dUnaG reporter fluorescence, and (j) unique spheroid HRE-dUnaG fluorescence in function of ki67 status (N = 3, n = 8). All data represent mean ± s.d., and each datapoint represents a single spheroid (b, d, e, j). P-values were determined with two-tailed Mann-Whitney test (b,g,i,j) or Kruskal-Wallis test followed by Dunn's post-hoc test (c, d). ns, not significant. **p < 0.01; ****p < 0.0001.