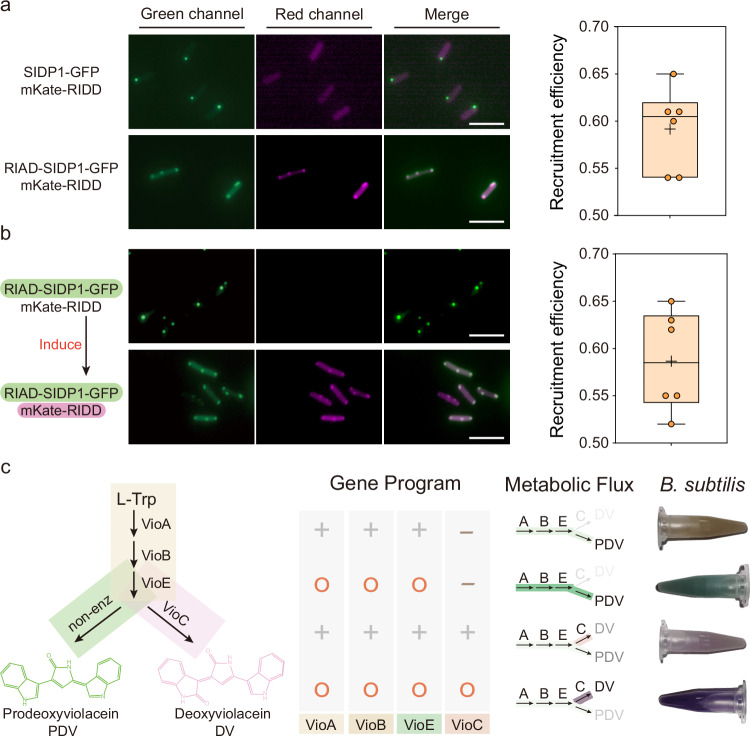
Fig. 3. Engineered intracellular condensates with intended function.
a Measuring recruitment efficiency of target fluorescent protein within condensates upon co-expression of RIAD-SIDP1-GFP and mKate-RIDD (n = 6 biologically independent cells). SIDP1-GFP protein without RIAD peptide was used as a negative control. b Measuring recruitment efficiency of mKate-RIDD (driven by xylose operon) within RIAD-SIDP1-GFP formed condensates (driven by IPTG). The first three columns indicate the dual-channel imaging and merging images of RIAD-SIDP1-GFP and mKate-RIDD. The fourth column represents the ratio of mKate fluorescence intensity of condensates to that of the whole cell (n = 6 biologically independent cells). The box plots in (a) and (b) show the mean value as a black cross, the center as a black line, box extending between the 25th and 75th percentiles, and whiskers indicating minimum and maximum data points. c Validation of the potential function of cellular condensates for controlling metabolic flux. The leftmost column represents the metabolic pathways of deoxyviolacein (DV) and prodeoxyviolacein (PDV). Cellular condensate programs flexibly redirect the metabolic flux of the DV/PDV biosynthesis pathway, resulting in a significant change in cell color. 1.5 mL culture were harvested by centrifugation. The “+” represents the gene being expressed in the strain, while the “-” indicates non-expression. Additionally, the “O” signifies that the protein is colocalized within the cellular condensate. Scale bars = 5 μm. Source data provided as a Source Data file.