Abstract
Biosynthesis and folding of multidomain transmembrane proteins is a complex process. Structural fidelity is monitored by endoplasmic reticulum (ER) quality control involving the molecular chaperone calnexin. Retained misfolded proteins undergo ER-associated degradation (ERAD) through the ubiquitin-proteasome pathway. Our data show that the major degradation pathway of the cystic fibrosis transmembrane conductance regulator (CFTR) with F508del (the most frequent mutation found in patients with the genetic disease cystic fibrosis) from the ER is independent of calnexin. Moreover, our results demonstrate that inhibition of mannose-processing enzymes, unlike most substrate glycoproteins, does not stabilize F508del-CFTR, although wild-type (wt) CFTR is drastically stabilized under the same conditions. Together, our data support a novel model by which wt and F508del-CFTR undergo ERAD from two distinct checkpoints, the mutant being disposed of independently of N-glycosidic residues and calnexin, probably by the Hsc70/Hsp70 machinery, and wt CFTR undergoing glycan-mediated ERAD.
Biosynthesis of functional proteins aimed at the cell surface involves transport through a series of membranous compartments, the first of which is the endoplasmic reticulum (ER), where they encounter the appropriate environment for folding, oligomerization, maturation, and export from the ER. However, folding of such proteins, particularly of multidomain transmembrane proteins, in the highly crowded macromolecular environment of the cell is a complex process. The export of these proteins from the ER to the Golgi is tightly coupled to the acquisition of a native conformation, as proteins that fail to fold properly, or that do not form the correct oligomeric structures, are recognized as abnormal, leading to ER retention, retrotranslocation, and ultimately cytoplasmic degradation by the ubiquitin (Ub)-proteasome pathway. This phenomenon has been described as ER quality control (ERQC) and is believed to be a characteristic of all eukaryotic cells (for a review, see reference 10). It was first postulated as the calnexin cycle (16) and later described as similarly involving calreticulin for lumenal ER substrates (38). ERQC functions as a protective mechanism, since it prevents abnormal proteins from clogging the secretory pathway and/or the accumulation of nonfunctional molecules at the cell surface (10).
The mechanism starts when oligosaccharyltransferase adds to newly synthesized proteins with Asn-X-Ser/Thr consensussequences that are cotranslationally inserted into the ER a branched 14-unit oligosaccharide (a process described as N glycosylation) containing two N-acetylglucosamines (N-AcGlc), nine mannoses (Man), and three glucoses (Glc). This glycan moiety is first recognized by glucosidases I and II (GI and GII, respectively), which trim the three glucose residues sequentially, and the intermediate monoglucosylated oligosaccharide structure is then recognized by the lectins calnexin and/or calreticulin, which also play a role as molecular chaperones by interacting with the attached polypeptidic chain of the client protein to promote its folding (10). Dissociation from calnexin-calreticulin coincides with trimming of the third glucose residue by GII. If the polypeptide is folded at this stage, it proceeds to the secretory pathway. However, misfolded glycoproteins are specifically recognized as such by UDP-glycoprotein glucosyltransferase, which reglucosylates them (37). This makes the glycoprotein substrate once more recognizable by calnexin-calreticulin, and it undergoes rounds of binding to these lectins, folding, and de- and reglucosylation, remaining in the ER until it is folded. There are thus only two ways out of the ERQC. The first, if the protein is folded, is through ER exit sites, by binding to specific cargo receptor molecules for sorting into COPII-coated vesicles destined for the Golgi apparatus. As an alternative way out, in the case of prolonged association of misfolded substrate with calnexin-calreticulin, the protein is sent for proteasomal ER-associated degradation (ERAD) (10). It is generally accepted that ERAD involves retrotranslocation, i.e., the export of glycoproteins from the lumen or the membrane of the ER into the cytoplasm through a translocation pathway, somewhat similar to the “forward pathway” involving the Sec61 translocation complex, before proteins to be degraded become substrates of the Ub-proteasome system (47). Although the mechanism of substrate delivery to the translocon is still unclear, there is recent evidence that N-linked glycans play a role in the selective targeting of misfolded glycoproteins to ERAD through interaction with the lectin EDEM (ER degradation-enhancing α-mannosidase-like protein; Htm1/Mnl1p in yeast) (21). Such a mechanism proposes that the selection of misfolded glycoproteins among folding intermediates is based on the length of time that the glycoprotein spends in the ER and that the glycoprotein substrate is marked for degradation upon mannose trimming of its N-linked glycan moiety (for reviews, see references 5 and 17). In the case of prolonged acquisition of a folded conformation, the glycoprotein GlcNAc2-Man9-(Glc1) oligosaccharide becomes a substrate of ER mannosidase I that specifically removes the terminal mannose from the middle branch (branch B), transforming it into GlcNAc2-Man8-Glc1 (also called Man8B-isomer), which functions as the predicted proximal signal for translocon delivery and ERAD (5).
Accordingly, EDEM was shown to accelerate the degradation of misfolded glycoprotein substrates when overexpressed (32, 35).
Although it has been clearly demonstrated that the N-linked glycan plays a role in the proper folding and quality control of at least some glycoproteins in eukaryotes from yeast to mammals, the diversity of ERAD substrates makes it hard to envision the existence of a unique degradative pathway. Indeed, recently multiple mechanisms have been reported to target different substrate proteins through distinct degradative pathways (4, 27, 44, 50). Among the alternative degradation pathways, some are described as independent of calnexin but involving the ER chaperone calreticulin (4) or BiP (44). Another alternative pathway was found to be independent of cytosolic components but sensitive to either tyrosine phosphatase blockage or ER mannosidase I inhibition (27). Examples of substrates following such alternative pathways are mutant variants of α1-antitrypsin (4), carboxypeptidase Y (27), and tyrosinase (44, 50). Other degradative pathways of glycoproteins may involve rhomboid intramembrane proteins (48) or a yet-uncharacterized proteasome-independent and nonlysosomal pathway (27).
Recently, the concept of a multiple checkpoint surveillance mechanism operating simultaneously for the quality control of glycoproteins has emerged (50). Moreover, based on data from glycoproteins containing distinct misfolded domains, it was suggested that it is the site of the lesion that determines the degradative pathway of the protein substrate (after ER-to-Golgi transport if on a lumenal domain or through the static retention of ERAD substrates in the ER) when lesions occur in a cytosolic domain (20, 50). This proposed mechanism, together with the apparent contradiction as to whether calnexin promotes or prevents the degradation of bound glycoprotein substrates (2), indeed suggests that multiple pathways may operate in the degradation of secretory proteins.
The cystic fibrosis (CF) transmembrane conductance regulator (CFTR) protein, a product of the gene that is mutated in the genetic disease CF, is a polytopic integral membrane protein, a member of the ABC transporter superfamily, with two nucleotide binding domains (NBD1 and -2), two membrane-spanning domains (1 and 2), and a highly hydrophobic large regulatory (R) domain reported to possess multiple phosphorylation sites (6). CFTR is localized at the apical membrane of epithelial cells, where it functions as a chloride (Cl−) channel regulated by ATP and protein kinase A (6). CFTR processing depends on the cell type (49), but it can be a rather inefficient process, as in some heterologous expression systems only ∼25 to 30% of synthesized wild-type (wt) CFTR reaches its correct location at the plasma membrane (53), in contrast to other ABC transporters, like MDR or MRP (26). For the most common CF-causing variant, deletion of phenylalanine 508 (F508del) in the amino-terminal NBD1, the process is even less efficient, as the protein is unable to fold correctly and to traffic to the cell membrane, being mostly retained in the ER, where it is targeted for Ub-proteasomal degradation (22, 23, 40, 54).
As CFTR synthesis is initiated at the ER, the nascent polypeptidic chain is associated with the cytosolic molecular chaperones Hsp90 (26), Hsc70/Hdj-2 (30), and Hsp70/Hdj-1 (12), and following N glycosylation (at asparagine residues 894 and 900), it also interacts with calnexin (39). Calnexin was previously shown to form complexes with both wt and F508del-CFTR, but the latter were found to be more stable (39), suggesting a role for this lectin chaperone in the ER retention of the mutant. Other authors, using a viral expression system to overexpress calnexin, reported that it increases the pool of F508del-CFTR at the ER and suggested a possible mechanism through the attenuation of ERAD (36). More recently, a study describing curcumin, an inhibitor of ER calcium pumps, as an effective agent to correct the F508del-CFTR trafficking defect proposed that the mechanism involved occurs through the partial inactivation of calnexin caused by a decrease in intracellular calcium levels (9). Although apparently discrepant, as for other substrates (2), these data suggest that calnexin has some function in the ERQC of CFTR, but a direct role of calnexin in folding and/or degradation of CFTR has not been demonstrated.
In this study, we looked at the in vivo involvement of calnexin and of the N-glycan moiety of CFTR on its ERQC (folding and degradation). For that purpose, we modulated calnexin levels inside the cell and treated CFTR-expressing cells with different inhibitors of enzymes responsible for processing of the glycan moiety. Moreover, we genetically engineered both wt CFTR and F508del-CFTR in order to replace the consensus N glycosylation residues and studied the degradation and processing of the resulting glycosylation-incompetent proteins.
MATERIALS AND METHODS
Site-directed mutagenesis.
Replacement of asparagine residues at positions 894 and 900 (corresponding to N-glycosylation sites) with alanine and glutamine residues (mutants N894,900A and N894,900Q, respectively) was performed using the QuickChange Site Directed Mutagenesis kit (Stratagene, La Jolla, CA). pNUT-wt-CFTR and pNUT-F508del-CFTR were used as templates for PCR mutagenesis. All sequences were confirmed by automatic DNA sequencing (ABI Prism 3100; Applied Biosystems, Foster City, CA).
Cell culture and transfections.
Chinese hamster ovary (CHO) and baby hamster kidney (BHK) cell lines stably transfected with wt or F508del-CFTR were cultivated as described previously (12). The nontransfected BHK cell line was cultivated as previously described (12). Stable transfections of BHK cells with CFTR mutants N894,900Q and N894,900A were performed as described previously (41).
For transient transfections, 5 μg of the human calnexin cDNA cloned into the AprM8 vector (18) was used with lipofectin to transfect CHO cells stably expressing wt or F508del-CFTR. A similar amount of the nonrecombinant AprM8 (produced by religation of the purified fragment after hydrolysis to remove calnexin cDNA) was used in mock transfections as a control. For double transfections with EDEM and calnexin, 3 μg of each expression plasmid was used. EDEM cDNA was cloned into the pCMV2Sport vector (Invitrogen, Carlsbad, CA).
In experiments with glycosidase inhibitors, purchased from Sigma (St. Louis, MO) or Calbiochem (San Diego, CA) unless otherwise stated, 90-min treatments were applied. Doses were as follows: 1-deoxymannojirimycin (DMM), 1 mM (46); castanospermine (CAS), 1 mM (46); kifunensine (KIF), 100 μM (4); swainsonine (SWN), 100 μM (4); N-butyldeoxynojirimycin (DNJ), 1 mM (4); MG132 (from Affiniti, Devon, United Kingdom), 25 μM; and cycloheximide (CHX), 20 μM (applied only during the chase period).
RNAi transfection.
RNA interference (RNAi) primers were designed as described elsewhere (http://www.mpibpc.gwdg.de/abteilungen). We used the 21-nucleotide sense strand 5′-AAAUCAGAUUCCAGUACUCCU-3′, corresponding to region 142 to 163 of hamster calnexin cDNA (GenBank accession number AY100687), synthesized by MWG Biotech. Small interfering RNA duplex was used at a concentration of 20 μM.
CHO cells stably expressing wt or F508del-CFTR were transfected using Oligofectamine reagent (Invitrogen) with 20, 60, or 120 pmol of calnexin RNAi heteroduplexes. Green fluorescent protein RNAi heteroduplexes were used as controls. Cells were lysed 24 h, 48 h, and 72 h posttransfection, and expression levels were assessed by Western blotting with anticalnexin antibody (Ab), as described below. For further studies, we used cells transfected with 60 pmol of RNAi primers for 24 h.
Antibodies.
The following Abs were used: mouse monoclonal anti-CFTR M3A7 Ab, generated against CFTR amino acids 1197 to 1480 (Chemicon, Temecula, CA; MAB3480); rabbit polyclonal anti-CFTR Ab, generated against a glutathione S-transferase fusion protein containing CFTR residues 1 to 79; monoclonal anti-calnexin AF8 antibody (18); polyclonal anti-calnexin carboxyl terminus (StressGen, Victoria, BC, Canada; SPA-860); and polyclonal anti-EDEM ER1 and ER2 antibodies (35).
Western blotting.
To assay for calnexin expression, cells stably expressing wt CFTR or F508del-CFTR were transfected as described above. The cells were lysed, and samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose filters as described previously (12). The filters were probed with the anti-human calnexin specific AF8 monoclonal antibody or anti-calnexin polyclonal antibody. Blots were developed using the ECL detection system (Amersham Biosciences, Uppsala, Sweden).
Pulse-chase and immunoprecipitation.
Twenty-four hours posttransfection, or after the desired period of incubation with the corresponding drug, cells expressing wt or F508del-CFTR were starved for 30 min in methionine-free medium and then pulsed for 30 min in the same medium supplemented with 150 μCi/ml [35S]methionine. After being chased for different time periods in medium supplemented with fetal bovine serum and 1 mM of nonradioactive methionine, the cells were lysed in 1 ml RIPA buffer and the immunoprecipitation (IP) was carried out as previously described (22), using either the polyclonal N-terminal Ab or the M3A7 monoclonal Ab and protein G-agarose beads. Immunoprecipitated proteins were eluted and electrophoretically separated on 7% (wt/vol) polyacrylamide gels (12). Fluorography, densitometry, and statistical analyses of data were performed as previously described (11, 12). Quantification of the core-glycosylated form of wt or F508del-CFTR at a given chase time was estimated as a percentage given by the ratio of the amount of the form (band B) at that chase time (P) over its amount at chase time zero (P0), i.e., at the end of the pulse period.
Sequential immunoprecipitations.
Cells were first lysed in chaperone buffer [50 mM Tris, pH 7.4, 150 mM NaCl, 10 mM (NH4)6Mo7O24, 0.09% (vol/vol) NP-40] and incubated overnight with the first Ab. Protein G-agarose beads (25-μl packed volume) were added, and incubation continued for a further 4-h period. After being washed with lysis buffer, the proteins were eluted from the beads in 1% (wt/vol) SDS for 1 h at 37°C (26). These eluates were then adjusted to the composition of RIPA lysis buffer for a second IP, which was carried out as described above.
Cell surface biotinylation and immunoprecipitation.
After treatment with CAS or DMM for 72 h (to account for recycling of all membrane CFTR), cell surface biotinylation was performed as described before (13). Briefly, cells were cooled down to 4°C, washed with phosphate-buffered saline (PBS) containing 1.0 mM MgCl2 and 0.1 mM CaCl2 (PBS c/m), and incubated for 30 min with sodium periodate (10 mM) in the dark. The cells were again washed with PBS c/m and labeled with 2 mM biotin-LC-hydrazide (Pierce) in 100 mM sodium acetate (pH 5.5) for 30 min. These labeled cells were washed with PBS c/m and lysed in 1 ml of RIPA buffer containing a cocktail of protease inhibitors. After biotinylation and lysis, samples were divided into two equal aliquots and IP was carried out with both anti-CFTR Ab and protein G-agarose. One of the IP aliquots was then eluted from the beads using Laemmli sample buffer without bromophenol blue and diluted 10-fold in RIPA buffer, and the biotinylated fraction was captured using avidin-Sepharose beads (Pierce). Both total CFTR and biotinylated CFTR were then in vitro phosphorylated using [γ-32 P]ATP (NEN) and cyclic AMP (cAMP)-dependent protein kinase (Promega) as described previously (13).
RESULTS
Turnover and processing of wt and F508del-CFTR after calnexin transient transfection.
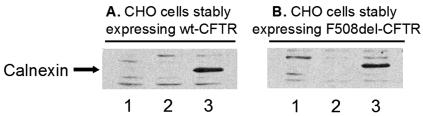
The effect of overexpressing calnexin upon CFTR turnover and processing was tested following transient transfection with calnexin cDNA. Calnexin was not detected by immunoblotting with an Ab specific for calnexin of human origin (see Materials and Methods) in non-calnexin-transfected or in mock-transfected CHO cells stably expressing either wt CFTR (Fig. 1A, lanes 1 and 2) or F508del-CFTR (Fig. 1B, lanes 1 and 2), but it was clearly detected in these cells collected 24 h posttransfection with AprM8-calnexin construct (Fig. 1A and B, lanes 3).
FIG. 1.
Detection of calnexin levels by Western blotting after transient transfection. CHO cells stably expressing either wt CFTR (A) or F508del-CFTR (B) were transiently transfected with human calnexin cDNA (see Materials and Methods). Lanes 1, nontransfected cells; lanes 2, mock-transfected cells (with the empty vector); lanes 3, cells analyzed 24 h posttransfection with calnexin cDNA. Blots were probed with an Ab specific for human calnexin, as indicated on the left.
Turnover and processing of wt and F508del-CFTR were thus studied at 24 h posttransfection with the calnexin construct by metabolic radiolabeling followed by IP with an anti-CFTR Ab.
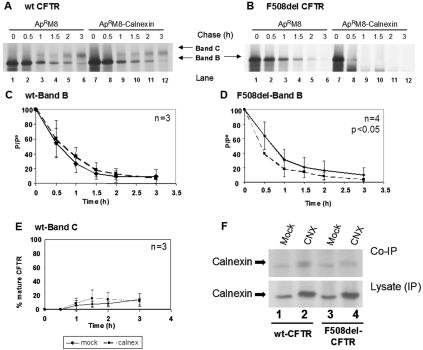
The decay rate of the immature form of wt CFTR was found not to be significantly altered after calnexin transfection relative to mock-transfected cells (Fig. 2A, compare the disappearance of band B in lanes 7 to 12 with lanes 1 to 6, and C).
FIG. 2.
Turnover and processing of wt and F508del-CFTR under calnexin overexpression. CHO cells stably expressing (A) wt or (B) F508del-CFTR were transiently transfected with the calnexin cDNA construct (lanes 7 to 12) or with the same amount of empty vector as a control (lanes 1 to 6). Twenty-four hours posttransfection, the cells were pulse-labeled for 30 min with [35S]methionine and chased for 0 h (lanes 1 and 7), 0.5 h (lanes 2 and 8), 1 h (lanes 3 and 9), 1.5 h (lanes 4 and 10), 2 h (lanes 5 and 11), and 3 h (lanes 6 and 12). The cells were then lysed and immunoprecipitated with an anti-CFTR Ab (see Materials and Methods). Following electrophoretic separation and fluorography, immature (band B) and mature (band C) forms of CFTR were quantified (see Materials and Methods). Turnover of the core-glycosylated form (band B) of wt CFTR (C) and F508del-CFTR (D) is shown as the ratio between P, the amount of band B at time t, and P0, the amount of band B at the start of the chase (i.e., at the end of pulse). The efficiency of conversion of the core-glycosylated form (band B) into the fully glycosylated form of wt CFTR (band C) was also estimated for wt CFTR (E) and was determined as the ratio between the amount of band C at time t and the amount of band B at the start of the chase (P0). The number of experiments is indicated at the right upper corner of panels C, D, and E. Statistically significant differences are indicated (P < 0.05). (F) (Top) Calnexin cDNA was used to transfect cells stably expressing wt or F508del-CFTR. After being labeled with [35S]methionine, the cells were lysed and CFTR immunoprecipitated with an anti-CFTR Ab. After elution, a second IP was performed (see Materials and Methods) using a mixture (1:1) of human and hamster anticalnexin Abs. Lanes 1 and 3, cells transfected with empty vector (as a control); lanes 2 and 4, cells transfected with calnexin cDNA. (Bottom) Results of direct IP of calnexin in lysates.
The turnover rate of core-glycosylated F508del-CFTR, however, is increased after calnexin transfection (Fig. 2B, compare the disappearance of band B in lanes 7 to 12 with lanes 1 to 6, and D). This increased turnover rate was found to be statistically significant in results from four independent experiments (12).
As for the efficiency of wt CFTR maturation, no significant change occurs under calnexin overexpression (Fig. 2A, compare band C in lanes 7 to 12 with lanes 1 to 6, and E). Similarly to non-calnexin-transfected cells, no maturation of F508del-CFTR is observed after calnexin transfection (Fig. 2B, no band C in lanes 7 to 12). Therefore, the increased turnover observed for band B of F508del-CFTR does not correspond to an increase in its processing efficiency, so we conclude that it results from an increased degradation rate.
Direct interaction between calnexin and CFTR was assessed by sequential IP after mock and calnexin transfections (Fig. 2F). The results show that calnexin associates with both wt and F508del-CFTR in either mock- or calnexin-transfected cells (Fig. 2F) and that the observed levels are increased by approximately twofold in the latter cells (Fig. 2F, top, compare lanes 1 and 3 to lanes 2 and 4).
Turnover and processing of wt and F508del-CFTR under down-regulation of calnexin levels by RNAi.
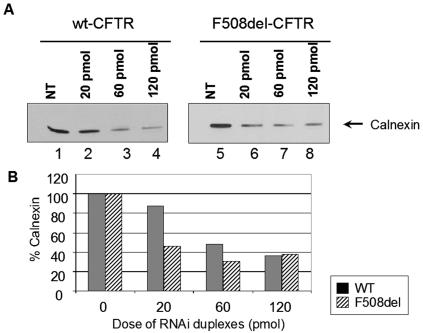
In order to study CFTR turnover and maturation under reduced calnexin levels, the same cells were transfected with RNAi duplexes specific for hamster calnexin mRNA (see Materials and Methods). Calnexin levels were assayed by immunoblotting as before, now using an Ab detecting hamster calnexin, after transfection with three different doses of RNAi (20, 60, and 120 pmol) at different times posttransfection (24 h, 48 h, and 72 h).
Figure 3A shows a significant decrease (60 to 70%) in the levels of endogenous calnexin 24 h after RNAi transfection (lanes 3 and 7 to 60 pmol of RNAi duplex and lanes 4 and 8 to 120 pmol, in comparison with nontransfected controls in lanes 1 and 5). Calnexin levels after 48 h were very similar to those observed for 24 h posttransfection. At 72 h posttransfection, cell viability was severely decreased (data not shown). Figure 3B shows the same results graphically expressed as the percentage of endogenous calnexin present 24 h after transfection with different amounts of RNAi duplexes. We chose the dose of 60 pmol of RNAi duplexes and 24 h as the time posttransfection to study turnover and maturation of CFTR as the best compromise between cell viability and decrease of calnexin levels.
FIG. 3.
Immunodetection of calnexin levels after transfection with RNAi duplexes specific for this chaperone. (A) CHO cells stably expressing wt CFTR (lanes 1 to 4) or F508del-CFTR (lanes 5 to 8) were transfected with calnexin RNAi duplexes (see Materials and Methods) and analyzed 24 h afterwards. The cells were lysed, and 30 μg of total protein was loaded onto an SDS-PAGE gel. Western blotting was performed using with an Ab recognizing endogenous (hamster) calnexin. Lanes 1 and 5, nontransfected (NT) cells; lanes 2 to 4 and 6 to 8, cells transfected with different amounts of RNAi duplexes (lanes 2 and 6, 20 pmol; lanes 3 and 7, 60 pmol; and lanes 4 and 8, 120 pmol). The arrow indicates detection of calnexin. (B) Blots were scanned, and densitometry was performed for quantification. The results are shown as a plot of the percentage of calnexin present relative to nontransfected cells.
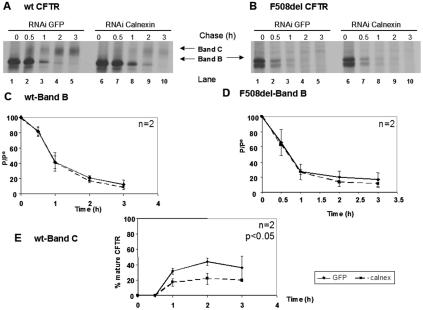
The results in Fig. 4 show that the turnover of either immature wt CFTR (Fig. 4A, band B in lanes 6 to 10, and C) or immature F508del-CFTR (Fig. 4B, band B in lanes 6 to 10, and D) is not significantly altered compared to the decay rates of these immature forms in the same cells treated with control RNAi duplexes (lanes 1 to 6 in Fig. 4A and B, respectively).
FIG. 4.
Turnover and processing of wt and F508del-CFTR under calnexin down-regulation by RNAi. CHO cells stably expressing (A) wt or (B) F508del-CFTR were transfected with 60 pmol of RNAi primers specific for calnexin (lanes 6 to 10) or green fluorescent protein RNAi primers as a negative control (lanes 1 to 5). Twenty-four hours posttransfection, the cells were pulse-labeled and chased as before (Fig. 2) for 0 h (lanes 1 and 6), 0.5 h (lanes 2 and 7), 1 h (lanes 3 and 8), 2 h (lanes 4 and 9), and 3 h (lanes 5 and 10). The cells were then lysed, immunoprecipitated with an anti-CFTR Ab, and analyzed as before (see the legend to Fig. 2) to determine the turnover of immature wt CFTR (C) and F508del-CFTR (D) and the processing efficiency of wt CFTR (E). The number of experiments and statistically significant differences are indicated as in Fig. 2.
However, the efficiency of processing of immature wt CFTR into band C evidenced a decrease (20 to 30%) under reduced calnexin levels (Fig. 4A, compare bands C in lanes 8 to 10 with lanes 3 to 5, and E). Although apparently modest, this decrease was shown to be statistically significant, suggesting a role for calnexin in the folding of wt CFTR and in its successful ER-to-Golgi export.
Turnover and processing of wt and F508del-CFTR under castanospermine and 1-deoxymannojirimycin.
In order to further clarify the roles of calnexin and of N-linked glycans in CFTR degradation and maturation, we treated cells with compounds that affect the trimming of N-linked oligosaccharides and hence also the interaction of substrate glycoproteins with calnexin. CAS, an indolizidine alkaloid, is a potent competitive inhibitor of both α- and β-glucosidases, thus inhibiting both GI and GII glycoprotein processing. Treatment with CAS therefore causes the accumulation of N-linked glycoproteins containing mostly oligosaccharides of the N-AcGlc2Man7-9Glc3 type (46). This inhibitory effect prevents glycoproteins from interacting with calnexin that recognizes only the monoglucosylated moiety (see the introduction).
DMM is described as a potent noncompetitive inhibitor of mannosidase I, blocking the synthesis of complex N-linked oligosaccharides (which require prior trimming of mannose residues) and causing the accumulation of glycoproteins bearing N-AcGlc2Man9 structures. This inhibitory effect has been reported to force glycoproteins to interact with calnexin (46).
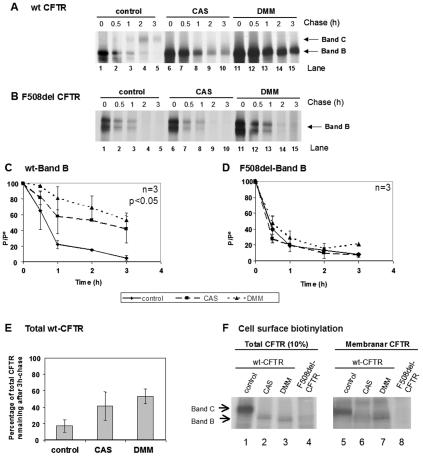
Turnover of the immature form of wt CFTR (band B) is significantly decreased in cells treated with 1 mM CAS for 90 min (Fig. 5A, lanes 6 to 10) relative to its decay in untreated cells (Fig. 5A, lanes 1 to 5, and C). In contrast, analysis of F508del-CFTR revealed that the turnover of the immature form (band B) is not significantly altered compared to the decay of this form in untreated cells (Fig. 5B, compare lanes 6 to 10 with 1 to 5, and D). As CAS inhibits processing of glycoconjugates (i.e., Golgi complex glycosylations), band C is not observed for wt CFTR under treatment with this compound.
FIG. 5.
Turnover of wt and F508del-CFTR under CAS and DMM. CHO cells stably expressing (A) wt CFTR or (B) F508del-CFTR were treated with 1 mM CAS (lanes 6 to 10) or 1 mM DMM (lanes 11 to 15) or untreated (lanes 1 to 5). After a 90-min treatment, the cells were pulse-labeled and chased for 0 h (lanes 1, 6, and 11), 0.5 h (lanes 2, 7, and 12), 1 h (lanes 3, 8, and 13), 2 h (lanes 4, 9, and 14), and 3 h (lanes 5, 10, and 15). The cells were then lysed, immunoprecipitated with an anti-CFTR Ab, and analyzed as before (see the legend to Fig. 2) to determine the turnover of immature wt CFTR (C) and F508del-CFTR (D). (E) Percentage of total CFTR (band B plus band C) remaining at the end of the chase period in untreated cells compared with total band B in cells treated with either CAS or DMM. The number of experiments and statistically significant differences are indicated as in Fig. 2. (F) Cell surface biotinylation followed by CFTR IP was performed to determine whether protein produced under CAS or DMM reaches the cell surface. CHO cells expressing wt or F508del-CFTR were incubated for 72 h with CAS or DMM. Cell surface proteins were biotinylated, and CFTR IP was carried out. Samples were divided into two equal portions, and biotin-labeled proteins were captured with streptavidin beads in one of the fractions. Total and biotinylated (membrane) CFTRs were then in vitro phosphorylated using [γ-32P]ATP and cAMP-dependent protein kinase. Ten percent of total immunoprecipitated CFTR (lanes 1 to 4) and all immunoprecipitated membrane CFTR (lanes 5 to 8) were subjected to SDS-PAGE and fluorography. Band B of wt CFTR produced under CAS or DMM could be clearly detected in the membrane fraction.
Our results in Fig. 5 show that, under DMM, the immature form of wt CFTR is drastically stabilized (Fig. 5A, compare lanes 11 to 15 with 1 to 6, and C). In contrast, analysis of immature F508del-CFTR under DMM revealed that its turnover is not significantly altered by DMM (Fig. 5B, compare lanes 11 to 15 with 1 to 6, and D).
We next determined whether the accumulation of wt CFTR band B observed under CAS and DMM results from a net stabilization effect (i.e., inhibition of a degradative pathway) or simply from the inhibition of Golgi processing (since protein that was otherwise observed as band C is now visualized as band B due to the lack of complex glycosylation). We thus estimated the difference between the amount of band B detected after a 3-h chase under CAS or DMM and the total CFTR (band B plus band C) detected after the same period without any treatment.
The graph in Fig. 5E clearly shows that the total amount of wt CFTR detected under either CAS or DMM treatment is significantly higher than that of total protein under control conditions. Thus, the observed accumulation does not result from just a block in processing. Indeed, more CFTR band B is detected under CAS or DMM (25% and 36%, respectively) than the total CFTR (band B plus band C) present in cells under control conditions. We therefore conclude that a real stabilization, i.e., inhibition of degradation, occurs under these treatments and thus a substantial proportion of wt CFTR degradation requires interaction with calnexin and mannose trimming of its glycan moiety. In contrast, since for F508del-CFTR no accumulation of the immature form was observed, we conclude that neither CAS nor DMM causes inhibition of its degradation.
In order to determine whether wt CFTR band B goes to the membrane under CAS or DMM treatment, we carried out the cell surface biotinylation assay (Fig. 5F). After treating cells expressing wt CFTR for 72 h with either CAS or DMM, glycoproteins present at the cell surface were biotinylated and CFTR was immunoprecipitated. CFTR present at the surface, and hence biotinylated, was then isolated from the immunoprecipitates with streptavidin beads.
The results from these biotinylation assays show that band B of wt CFTR produced under CAS or DMM (and thus lacking processed glycan structures) is present at the cell surface (Fig. 5F, lanes 6 and 7).
Inhibition of F508del-CFTR degradation.
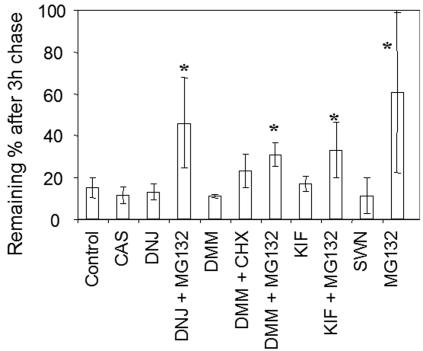
To further clarify this failure of CAS and DMM in stabilizing F508del-CFTR, we tested whether inhibitors of different glycosidases, proteasome, or translation, alone or combined, cause stabilization of this mutant protein. Cells were preincubated, labeled, and chased for 3 h in the presence of each of the following compounds: CAS (see above); DNJ, another inhibitor of both GI and GII (4); DMM (see above); KIF, a specific inhibitor of ER-mannosidase I (4); SWN, an inhibitor of mannosidase II (4), used alone or combined with CHX, an inhibitor of protein synthesis; and the proteasome inhibitor MG132 (22) alone or combined with some of the previous compounds. The results were plotted as percentages of CFTR protein remaining after a 3-h chase and incubation with different compounds (Fig. 6).
FIG. 6.
Degradation of F508del-CFTR under various inhibitors. CHO cells stably expressing F508del-CFTR were treated with 1 mM CAS, 1 mM DMM, 100 μM KIF, 100 μM SWN, 1 mM DNJ, 25 μM of proteasome inhibitor MG132, or 20 μM of the translation elongation inhibitor CHX, alone or simultaneously. After 90 min of treatment, the cells were pulse-labeled and chased as before (Fig. 2), but at a single time point (3 h). The cells were then lysed, immunoprecipitated with an anti-CFTR Ab, and analyzed as before (see the legend to Fig. 2) to determine the percentage of F508del-CFTR remaining after chase. The obtained data are shown graphically. The asterisk indicates statistically significant differences relative to the control (first bar).
Stabilization of F508del-CFTR was evaluated as the difference between total CFTR present under control conditions and in the presence of each of the above inhibitors. Significant stabilization was observed either when treatment with DNJ, DMM, or KIF was combined with MG132 or when the proteasome inhibitor was used alone (Fig. 6). Although to a lesser extent, stabilization was also observed after incubation with DMM combined with CHX. The highest levels of stabilization achieved, however, were only ∼50% of the initial amount of CFTR produced. None of the other conditions tested (CAS, DNJ, DNJ-MG132, DMM, DMM-CHX, KIF, or SWN) induced significant stabilization of the mutant protein. We therefore conclude that degradation of F508del-CFTR is not significantly mediated by its glycan moieties.
Stability of nonglycosylated CFTR.
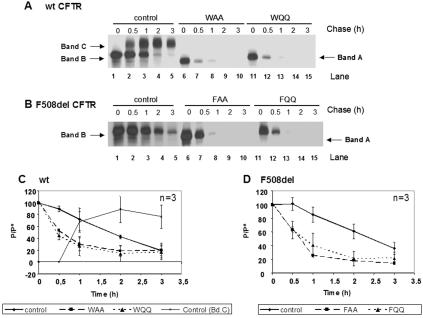
Because some of the inhibitors used here have been reported to inhibit glycosidases other than those for which they were initially reported to be specific, we also studied the impact of N glycosylation on wt and F508del-CFTR turnover using a genetic approach. As the N-glyconjugate is attached to asparagine residues 894 and 900 of CFTR, we replaced both these residues by either glutamine (N894Q and N900Q) or alanine (N894A and N900A) residues and studied the CFTR turnover of the resulting proteins on wt and F508del backgrounds when stably expressed in BHK cells as above.
The results show that the turnover of wt CFTR with either two alanines (WAA) or two glutamines (WQQ) is significantly increased (Fig. 7A, compare lanes 6 to 10 and 11 to 16 with 1 to 5), most probably corresponding to an increase in the degradation rate of the protein (Fig. 7C). No band B or C (i.e., glycan-processed CFTR) is observed for these genetic variants due to the complete absence of N glycosylation (only band A, i.e., the primary polypeptidic chain of CFTR, is observed). Analysis of F508del-CFTR either with two alanines (FAA) or two glutamines (FQQ) revealed that the turnover of the two N glycosylation-deficient proteins is also significantly increased compared to cells expressing F508del-CFTR with the consensus asparagines residues (Fig. 7B, compare lanes 6 to 10 and 11 to 15 with 1 to 5, and D).
FIG. 7.
Turnover of nonglycosylated mutants of wt CFTR (WQQ and WAA) and F508del-CFTR (FQQ and FAA). BHK cells stably expressing wt CFTR (A, lanes 1 to 5), N894A-N900A (WAA) (A, lanes 6 to 10), N894Q-N900Q (WQQ) (A, lanes 11 to 15), F508del-CFTR (B, lanes 1 to 5), F508del-N894A-N900A (FAA) (B, lanes 6 to 10), or F508del-N894Q-N900Q CFTR (FQQ) (B, lanes 11 to 15) were pulse-labeled and chased as before (Fig. 2) for 0 h (lanes 1, 6, and 11), 0.5 h (lanes 2, 7, and 12), 1 h (lanes 3, 8, and 13), 2 h (lanes 4, 9, and 14), and 3 h (lanes 5, 10, and 15). The cells were then lysed, immunoprecipitated with an anti-CFTR Ab, and analyzed as before (see the legend to Fig. 2) to determine the turnover of wt, WAA, and WQQ (C) and F508del-, FAA, and FQQ (D) CFTRs. The number of experiments and statistically significant differences are indicated as in Fig. 2.
These data, although reinforcing the role of N glycosylation on the (calnexin-mediated) folding and overall stability of wt CFTR, also support the existence of a glycan-independent ERAD pathway for CFTR.
Involvement of EDEM in the degradation of CFTR.
In order to clarify the mechanism of CFTR degradation, we also investigated the possible involvement of EDEM. EDEM is a Man8B-binding protein that was previously shown to accelerate the degradation of misfolded client proteins in the ER like α1-antitrypsin, through its interaction with the transmembrane region of calnexin (32). More recently, the yeast EDEM orthologue, Htm1p/Mnl1, was implicated in CFTR degradation when expressed in this model organism (15).
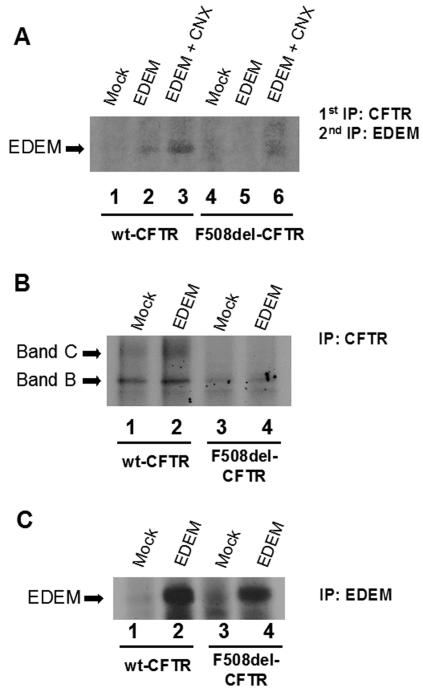
Cells expressing wt and F508del-CFTR were transiently transfected with the EDEM cDNA alone or cotransfected with calnexin cDNA. Sequential IP with anti-CFTR antibody followed by anti-EDEM was performed. The results in Fig. 8A show that when EDEM is overexpressed alone or with calnexin, it coprecipitates with wt CFTR (Fig. 8A, lanes 2 and 3). For F508del-CFTR, only a minor amount of EDEM associates with CFTR, but only when calnexin is overexpressed (Fig. 8A, lane 6).
FIG. 8.
Presence of EDEM in CFTR complexes. (A) EDEM cDNA was used alone or with calnexin (CNX) cDNAs to transfect cells stably expressing wt or F508del-CFTR. After being labeled with [35S]methionine, the cells were lysed and CFTR IP was performed with an anti-CFTR Ab. After elution, a second IP was performed using a specific anti-EDEM Ab. Lanes 1 and 4, cells transfected with empty vector (as a control); lanes 2 and 5, cells transfected with EDEM cDNA; lanes 3 and 6, cells cotransfected with EDEM and calnexin cDNAs. As controls, direct IPs of either CFTR (B) or EDEM (C) were also performed after transient transfection with EDEM cDNA.
As controls, direct IPs of CFTR and EDEM were also performed in mock- or EDEM-transfected cell lines stably expressing wt or F508del-CFTR. CFTR was detected both in mock- and EDEM-transfected cells (Fig. 8B). EDEM could be detected only by IP in wt and F508del-CFTR-expressing cells after transient transfection with EDEM cDNA (Fig. 8C).
DISCUSSION
Calnexin is a lectin chaperone that is able to bind newly synthesized client proteins to promote their folding, in part by preventing their incorrect aggregation in an unfolded intermediate conformation (3). It has been broadly implicated in the ERQC responsible for intracellular retention of misfolded or unassembled membrane glycoproteins (10, 16). Monitoring of the folding status of transmembrane proteins possessing large cytoplasmic domains, like CFTR, has also been described as largely dependent on the folding machines in the cytoplasm, namely, Hsc70/Hsp70 and Hsp90 (12, 26, 30, 56, 60). Although previous results from other authors have demonstrated the presence of calnexin in wt and F508del-CFTR complexes (36, 39), a clear role of this ER chaperone in CFTR folding and/or degradation has not been demonstrated. Here, we assessed the contribution of calnexin in determining the fate of wt and F508del-CFTR to clarify the mechanism by which the cell monitors the conformation status of this transmembrane protein.
Our results demonstrate that when calnexin is overexpressed, the turnover of the ER-specific immature form of wt CFTR (band B) is not affected, nor is the efficiency of its processing into its post-ER, mature form (band C) (Fig. 2). However, under the same conditions, our results show that the decay rate of the F508del mutant (band B) is significantly increased (Fig. 2), with no maturation (no band C) observed. We therefore, conclude that increased calnexin levels cause the mutant protein to be more rapidly degraded.
Although other authors (36) have reported that calnexin overexpression partially attenuates ERAD of F508del-CFTR, these data have to be interpreted with great care because the authors used adenoviral transfections to overexpress calnexin. Indeed, viral infections in general, and adenovirus in particular, are strong inducers of misfolded or abnormal cellular proteins, which constitute very potent triggers of the stress response in mammalian cells, namely, stress-induced molecular chaperones (55). Therefore, it is plausible to think that, besides calnexin, a number of other molecular chaperones (like Hsp70 and Hdj-1), whose levels were not assessed, are overexpressed under the experimental conditions used by those authors (36). These chaperones are known to stabilize a number of substrates, including CFTR (12).
Under down-regulated (by RNAi) levels of calnexin, however, the turnover of immature forms of either wt or F508del-CFTR is not affected (Fig. 4). Notwithstanding, the efficiency of processing of wt CFTR into band C is impaired (from 30% to 20%), in agreement with previously published data describing a decrease in steady-state levels of mature wt CFTR under calnexin down-regulation (36). These observations confirm the chaperone role of this lectin in the productive folding of wt CFTR. The fact that after the intracellular levels of calnexin are lowered no change is observed in the turnover of F508del-CFTR suggests that the major ERQC checkpoint for this mutant is not calnexin dependent (58).
In order to obtain further evidence for the (lack of) involvement of calnexin in the ERAD of F508del-CFTR and also to assess the impact of the glycan moieties on CFTR degradation, we performed studies of CFTR stability under various treatments with different glycosidase inhibitors, CAS and DMM in particular (Fig. 5). Treatment of mammalian cells with CAS, an inhibitor of GI and GII, has been reported to strongly stabilize several glycoprotein substrates, like apolipoprotein A, tyrosinase, and α1-antitrypsin (28, 51, 52). It is generally accepted that this effect results from inhibition of N-glycan trimming and consequent prevention of substrate protein interaction with calnexin. DMM, on the other hand, a general inhibitor of α-mannosidases, was reported to cause an enhancement of calnexin interaction with glycoproteins, resulting in a block in ERAD of these protein substrates. Numerous reports in the literature have supported this stabilizing effect of DMM on multiple protein substrates entering the secretory pathway (28, 52). Consistent with such data, our results show that treatments with either CAS or DMM cause a dramatic stabilization of wt CFTR (which is observed only as the core-glycosylated form, or band B, since both these inhibitors prevent Golgi processing of its glycan moiety). Some of this unprocessed wt CFTR, however, was found to be at the membrane (Fig. 5F). Other authors had previously detected a cAMP-mediated Cl− conductance in cells treated with DMM (34). Nevertheless, and as the total amount of wt CFTR obtained under either of these treatments is larger than the total of immature plus mature forms detected under physiological conditions (i.e., the sum of bands B and C), we conclude that both DMM and CAS bring about a net and effective stabilization of wt CFTR, i.e., they prevent wt CFTR delivery to ERAD.
Thus, data obtained under DMM are consistent with a model in which delivery of wt CFTR to ERAD is mediated by processing of its glycan moiety, possibly through the Man8B-mediated pathway, as suggested for other substrates (5). Stabilization of wt CFTR by CAS suggests that this degradative pathway is also calnexin dependent.
In striking contrast, F508del-CFTR is not stabilized when cells are treated with either of these drugs. As described above with calnexin RNAi studies, again these data strongly suggest that abrogating the interaction of F508del-CFTR with calnexin (CAS treatment) has no major (positive or negative) effect on ERAD of F508del-CFTR. Moreover, since inhibition of mannosidase I by DMM produces no stabilization effect on F508del-CFTR degradation, this excludes the glycan moiety (and the Man8B intermediate in particular) as the signal that targets most F508del-CFTR for ERAD, contrary to what was observed for several other glycoprotein substrates (5, 17) and here for wt CFTR.
Furthering an attempt to investigate how F508del-CFTR is degraded, we combined the use of several glycosidase inhibitors, namely, DNJ, equivalent to CAS as a blocker of GI and GII; KIF, an inhibitor reported to inhibit ER mannosidase I (the enzyme generating Man8 proteins) more specifically than DMM; and SWN, an inhibitor of mannosidase II. None of these compounds stabilizes the mutant protein (Fig. 6). Stabilization of F508del-CFTR was observed only when these glycosidase inhibitors were combined with either the proteasome inhibitor MG-132 or the inhibitor of translation CHX. By inhibiting the proteasome, we observed only about 50% CFTR stabilization, in accordance with what was previously described (22, 54) and suggesting possible alternative degradation pathways. On the other hand, the observed stabilization under CHX treatment, an inhibitor of protein synthesis that “freezes polysomes” that are actively involved in translation, suggests that degradation of F508del-CFTR is mediated by a labile protein. Alternatively, degradation of F508del-CFTR could be coupled to active translation.
These data lead us to propose that most F508del-CFTR is excluded from the calnexin-folding cycle, although some may actually get there (Fig. 2F). Most is probably sent for proteasomal degradation in an early intermediate conformational state and before interaction with calnexin. In fact, very recent data demonstrate that most F508del-CFTR is sent to premature degradation through an Hsc70-CHIP-Ub5H5a pathway while still bound to Hsc70 (57). However, as we show here, when calnexin is in excess, F508del-CFTR may be forced to interact with this lectin chaperone (Fig. 2F), resulting in the observed acceleration of its degradation rate. Similarly to what happens for other chaperones, namely, Hsp/Hsc70 (19, 29, 33), calnexin would thus have a dual role, i.e., one in the folding of wt CFTR and another in its ER disposal (and, if in excess, of F508del-CFTR, too).
Furthermore, together, our data for wt and F508del-CFTR strongly support a model in which most of each form of this protein undergoes different degradative pathways or is conformationally assessed at distinct checkpoints (see below).
To further investigate the roles of Asn N-linked glycans in the degradation of CFTR, we abrogated its two glycosylation sites. Asparagine residues at positions 894 and 900 were replaced by either two alanines (N894A and N900A) or two glutamines (N894Q and N900Q). Our results show that the N glycosylation null mutants of both wt and F508del-CFTR are significantly more unstable than their glycosylation-competent counterparts (Fig. 7). Such data support the important role of N glycosylation in the folding and in the overall stability of CFTR, as generally described (17), although they are inconclusive for the mechanism of ERAD of CFTR. Moreover, these data strongly support the existence of an alternative, i.e., glycan-independent ERAD pathway for transmembrane proteins.
Since wt CFTR seems to undergo glycan-mediated ERAD, the possible involvement of EDEM in the degradation pathway of CFTR was also investigated. EDEM is an ER transmembrane protein which when overexpressed accelerates degradation of misfolded α1-antitrypsin and the β-site amyloid precursor protein BACE457 (32). EDEM is reported to depend on ER mannosidase I activity to generate the Man8B moiety and is involved in the critical step that directs the misfolded glycoproteins carrying such a glycan structure to ERAD. More recently, it was shown that the targeting of proteins to ERAD by EDEM occurs when client proteins are still bound to calnexin (32).
Although we were able to detect EDEM in complexes of both wt and F508del-CFTR (only when calnexin is overexpressed simultaneously with EDEM), the latter was detected in reduced amounts in complexes of F508del-CFTR (Fig. 8). These results are consistent with wt CFTR being a substrate to the EDEM/glycan-mediated pathway (15) while further supporting its exclusion as a major substrate for F508del-CFTR degradation (14).
A partitioning between distinct disposal pathways was previously described for other substrates, like the PI Z variant α1-antitrypsin (27), for which it was proposed that misfolded mutant α1-antitrypsin is targeted for degradation at a different checkpoint that targets its misfolded wt counterpart for degradation. A similar mechanism may also exist for wt and F508del-CFTR.
Overall, the data shown here strongly support a model in which the ERQC of CFTR occurs via a two-step mechanism, i.e., with the protein conformationally assessed in the ER at least at two distinct checkpoints (Fig. 9). Accordingly, the major degradation pathway for F508del-CFTR would occur through a calnexin- and glycan-independent pathway and, we postulate, before it interacts with calnexin. This model is in accordance with two previous reports describing the pathways involving the E2-ubiquitin-conjugating enzymes Ubc6p (24) and Ubc5H5a (57) as major degradative pathways for F508del-CFTR, the latter coupled to the E3 CHIP via Hsc70 interaction.
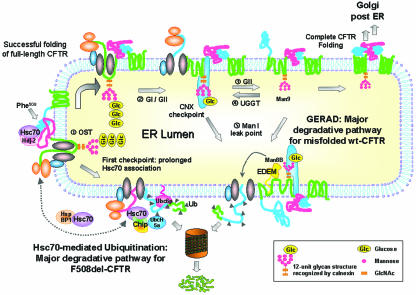
FIG. 9.
Proposed model for the major degradation pathways of F508del- and wt CFTR. (i) Synthesis of CFTR occurs with its concomitant insertion in the ER membrane and attachment of an Hsc70/Hdj-2 (or Hsp70/Hdj-1) pair to nascent cytosolic domains, as described previously (12, 30). Other authors have described increased levels of Hsc70/Hdj-2 complexes with F508del-CFTR relative to wt CFTR and expression of NBD1 as the earliest point at which Hsc70/Hdj-2 could bind the nascent CFTR polypeptidic chain (30). The same study reported that complex formation between Hdj-2 and nascent wt CFTR was greatly reduced after expression of the R domain, suggesting NBD1-R domain interaction as a critical point in CFTR folding. The cell thus seems to use this Hsc70/Hsp70 control as the first checkpoint to assess CFTR conformation, and we propose that it is the major mechanism to discard F508del-CFTR. Prolonged retention of unfolded F508del-CFTR by Hsc70 at this point enables CHIP to interact with Hsc70/Hsp70 (probably by displacing Hdj-2) and causes the mutant to be degraded through the Hsc70-CHIP-UbcH5a pathway (31, 57). The E2 Ubc6 may also contribute to F508del-CFTR ERAD (24). Contrary to what happens with F508del-CFTR, wt CFTR, for which NBD1-R intramolecular interaction and folding is achieved, proceeds in the folding pathway through interaction of its N-glycosyl residues (ii) with calnexin (iii). Wt CFTR acquires its native conformation through successive rounds of release-deglucosylation (iv) and rebinding-reglucosylation (v) to calnexin, which also constitutes the second ERQC checkpoint. Upon successful folding, CFTR exits the ER, proceeding through the secretory pathway (vi). However, prolonged presence in the calnexin cycle may cause misfolded CFTR to become a substrate of mannosidase I (vii). This enzyme trims mannose residues from the protein glycan moiety, possibly generating the Man8B glycan intermediate that is recognized by EDEM, which targets the client protein to ERAD (viii). We call this ER-degradative pathway GERAD, to indicate its dependence on the glycan moiety. According to this model, F508del-CFTR follows a major degradative pathway from the first (Hsc70-dependent) ERQC checkpoint, whereas misfolded wt CFTR undergoes proteolytic GERAD at the second (calnexin-dependent) one (see the text for a description).
Which features would retain F508del-CFTR at this first ER checkpoint and allow wt CFTR to proceed to the calnexin control? Although the exact structural determinants will probably remain unidentified until the fine structure of the full-length protein is known, wt CFTR has been reported to interact with and to have its translation facilitated by the chaperone Hsc70 alone (43) or with Hdj2 (30) or by Hsp70/Hdj1 (12), but it has also been reported to pass quickly through the Hsc70-mediated ERQC. We postulate that it proceeds to the calnexin checkpoint. In contrast, F508del-CFTR would be kinetically trapped at this first checkpoint. Indeed, it was shown that the earliest stage at which Hdj-2/Hsc70 could bind CFTR translation intermediates coincided with the biosynthesis of NBD1 (where F508 is localized) (30). Although it has recently been shown that Phe at position 508 sits at the surface of NBD1 (25), probably playing a limited role in the stabilization of the protein in its native state, it has been suggested that it makes crucial contacts during the folding process of the whole protein (40, 45). Indeed, formation of Hdj-2/Hsc70 complexes with nascent CFTR was shown to be greatly reduced after expression of the R domain. This suggests that the intramolecular NBD1-R-domain interaction, catalyzed by Hdj-2/Hsc70, is a critical step in the CFTR-folding pathway and that it is defective in the biogenesis of F508del CFTR (30). More recently, however, it was proposed that deletion of Phe508 also disrupts folding of NBD2 and the NBD1-NBD2 interaction (8).
Interestingly, CHIP, also an Hsc70 binding protein, is able to switch its chaperoning activity from protein folding to protein degradation by mediating the covalent attachment of ubiquitin (E3 ubiquitin ligase activity) to chaperone substrate proteins (7, 31). Indeed, CHIP was shown to induce degradation of immature CFTR by turning the Hsc70 molecular chaperone into a protein degradation factor (31). Prolonged association of a substrate with Hsc70 can give CHIP the opportunity to displace Hdj-2 from the carboxyl terminus of Hsc70, perhaps by competing or otherwise altering the affinity of Hdj-2 to bind Hsc70 (7), thus creating a kinetic trap that targets the substrate protein to degradation.
Recently, HspBP1, yet another cochaperone of Hsp70, was shown to act as an inhibitor of CHIP by attenuating its ubiquitin ligase activity when complexed with Hsc70 (1). Furthermore, it was shown that HspBP1 is able to rescue misfolded CFTR from CHIP-induced degradation while stimulating its maturation (1). In contrast, cysteine string protein (Csp), another J-domain-containing Hsc70-binding protein that also associates with membranes and is reported to stabilize the immature form of wt CFTR (59), could act by “freezing” its interaction with Hsc70, thus preventing both its interaction with other cochaperones and substrate release.
In summary, we propose a novel model (Fig. 9) in which the cell uses the first Hsc70-dependent checkpoint of ERQC to discard F508del-CFTR. One possibility is that a major difficulty in the folding of the mutant results from defective intramolecular NBD1-R-domain interaction that is detected cotranslationally by the Hsc70/Hsp70 machinery, which no longer releases the polypeptidic chain, enabling CHIP and degradation to take over Hdj-2 and folding-release (Fig. 9). In contrast, for wt CFTR, this intramolecular interaction would be achieved, and although a substantial amount of wt CFTR undergoes proteolytic degradation before being Golgi processed, the respective ERAD pathway involves its glycan moiety, calnexin, and probably EDEM. We call this Man8B-dependent ER-degradative pathway GERAD, to indicate its dependence on the glycan moiety. F508del-CFTR, due to prolonged retention by the Hsc70/Hsp70 machinery, which sends it for degradation at the first conformational checkpoint, would hardly be assessed at this second calnexin checkpoint. This model of ERQC for wt and F508del-CFTR does not exclude additional conformation checkpoints (namely, post-ER), which indeed have been proposed (42).
Acknowledgments
We are grateful to Luís Reis and Mário Neto for helping with the production of CFTR glycosylation mutants and iodide efflux assays, respectively. We thank K. Kirk (Birmingham, Ala.) for N-terminal anti-CFTR antibody, M. B. Brenner (Boston, Mass.) for the anti-calnexin AF8 antibody and cDNA, J. R. Riordan (Scottsdale, Ariz.) for the CHO cell lines stably expressing wt and F508del-CFTR, G. Lukacs (Toronto, Canada) for the BHK cell line stably expressing wt CFTR, and K. Nagata (Kyoto, Japan) for anti-EDEM antibody and cDNA.
This work was supported by Praxis XXI research grants POCTI/35737/MGI/2000 and POCTI/47382/MGI/2002 and Praxis Ph.D. fellowship (C.M.F.) BD/11094/97.
REFERENCES
- 1.Alberti, S., K. Bohse, V. Arndt, A. Schmitz, and J. Hohfeld. 2004. The cochaperone HspBP1 inhibits the CHIP ubiquitin ligase and stimulates the maturation of the cystic fibrosis transmembrane conductance regulator. Mol. Biol. Cell 15:4003-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayalon-Soffer, M., M. Shenkman, and G. Z. Lederkremer. 1999. Differential role of mannose and glucose trimming in the ER degradation of asialoglycoprotein receptor subunits. J. Cell Sci. 112:3309-3318. [DOI] [PubMed] [Google Scholar]
- 3.Bergeron, J. J., M. B. Brenner, D. Y. Thomas, and D. B. Williams. 1994. Calnexin: a membrane-bound chaperone of the endoplasmic reticulum. Trends Biochem. Sci. 19:124-128. [DOI] [PubMed] [Google Scholar]
- 4.Cabral, C. M., Y. Liu, K. W. Moremen, and R. N. Sifers. 2002. Organizational diversity among distinct glycoprotein endoplasmic reticulum-associated degradation programs. Mol. Biol. Cell 13:2639-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabral, C. M., Y. Liu, and R. N. Sifers. 2001. Dissecting glycoprotein quality control in the secretory pathway. Trends Biochem. Sci. 26:619-624. [DOI] [PubMed] [Google Scholar]
- 6.Collins, F. S. 1992. Cystic fibrosis: molecular biology and therapeutic implications. Science 256:774-779. [DOI] [PubMed] [Google Scholar]
- 7.Demand, J., S. Alberti, C. Patterson, and J. Hohfeld. 2001. Cooperation of a ubiquitin domain protein and an E3 ubiquitin ligase during chaperone/proteasome coupling. Curr. Biol. 11:1569-1577. [DOI] [PubMed] [Google Scholar]
- 8.Du, K., M. Sharma, and G. L. Lukacs. 2004. The DeltaF508 cystic fibrosis mutation impairs domain-domain interactions and arrests post-translational folding of CFTR. Nat. Struct. Mol. Biol. 12:17-25. [DOI] [PubMed] [Google Scholar]
- 9.Egan, M. E., M. Pearson, S. A. Weiner, V. Rajendran, D. Rubin, J. Glockner-Pagel, S. Canny, K. Du, G. L. Lukacs, and M. J. Caplan. 2004. Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science 304:600-602. [DOI] [PubMed] [Google Scholar]
- 10.Ellgaard, L., and A. Helenius. 2003. Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell. Biol. 4:181-191. [DOI] [PubMed] [Google Scholar]
- 11.Farinha, C. M., F. Mendes, M. Roxo-Rosa, D. Penque, and M. D. Amaral. 2004. A comparison of 14 antibodies for the biochemical detection of the cystic fibrosis transmembrane conductance regulator protein. Mol. Cell Probes 18:235-242. [DOI] [PubMed] [Google Scholar]
- 12.Farinha, C. M., P. Nogueira, F. Mendes, D. Penque, and M. D. Amaral. 2002. The human DnaJ homologue (Hdj)-1/heat-shock protein (Hsp) 40 co-chaperone is required for the in vivo stabilization of the cystic fibrosis transmembrane conductance regulator by Hsp70. Biochem. J. 366:797-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farinha, C. M., D. Penque, M. Roxo-Rosa, G. Lukacs, R. Dormer, M. McPherson, M. Pereira, A. G. Bot, H. Jorna, R. Willemsen, H. Dejonge, G. D. Heda, C. R. Marino, P. Fanen, A. Hinzpeter, J. Lipecka, J. Fritsch, M. Gentzsch, A. Edelman, and M. D. Amaral. 2004. Biochemical methods to assess CFTR expression and membrane localization. J. Cyst. Fibros. 3(Suppl. 2):73-77. [DOI] [PubMed] [Google Scholar]
- 14.Gelman, M. S., E. S. Kannegaard, and R. R. Kopito. 2002. A principal role for the proteasome in endoplasmic reticulum-associated degradation of misfolded intracellular cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 277:11709-11714. [DOI] [PubMed] [Google Scholar]
- 15.Gnann, A., J. R. Riordan, and D. H. Wolf. 2004. CFTR degradation depends on the lectins Htm1p/EDEM and the Cdc48 protein complex in yeast. Mol. Biol. Cell 15:4125-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammond, C., and A. Helenius. 1994. Quality control in the secretory pathway: retention of a misfolded viral membrane glycoprotein involves cycling between the ER, intermediate compartment, and Golgi apparatus. J. Cell Biol. 126:41-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helenius, A., and M. Aebi. 2004. Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 73:1019-1049. [DOI] [PubMed] [Google Scholar]
- 18.Hochstenbach, F., V. David, S. Watkins, and M. B. Brenner. 1992. Endoplasmic reticulum resident protein of 90 kilodaltons associates with the T- and B-cell antigen receptors and major histocompatibility complex antigens during their assembly. Proc. Natl. Acad. Sci. USA 89:4734-4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hohfeld, J., D. M. Cyr, and C. Patterson. 2001. From the cradle to the grave: molecular chaperones that may choose between folding and degradation. EMBO Rep. 2:885-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huyer, G., W. F. Piluek, Z. Fansler, S. G. Kreft, M. Hochstrasser, J. L. Brodsky, and S. Michaelis. 2004. Distinct machinery is required in Saccharomyces cerevisiae for the endoplasmic reticulum-associated degradation of a multispanning membrane protein and a soluble luminal protein. J. Biol. Chem. 279:38369-38378. [DOI] [PubMed] [Google Scholar]
- 21.Jakob, C. A., D. Bodmer, U. Spirig, P. Battig, A. Marcil, D. Dignard, J. J. Bergeron, D. Y. Thomas, and M. Aebi. 2001. Htm1p, a mannosidase-like protein, is involved in glycoprotein degradation in yeast. EMBO Rep. 2:423-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen, T. J., M. A. Loo, S. Pind, D. B. Williams, A. L. Goldberg, and J. R. Riordan. 1995. Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell 83:129-135. [DOI] [PubMed] [Google Scholar]
- 23.Kartner, N., O. Augustinas, T. J. Jensen, A. L. Naismith, and J. R. Riordan. 1992. Mislocalization of delta F508 CFTR in cystic fibrosis sweat gland. Nat. Genet. 1:321-327. [DOI] [PubMed] [Google Scholar]
- 24.Lenk, U., H. Yu, J. Walter, M. S. Gelman, E. Hartmann, R. R. Kopito, and T. Sommer. 2002. A role for mammalian Ubc6 homologues in ER-associated protein degradation. J. Cell Sci. 115:3007-3014. [DOI] [PubMed] [Google Scholar]
- 25.Lewis, H. A., S. G. Buchanan, S. K. Burley, K. Conners, M. Dickey, M. Dorwart, R. Fowler, X. Gao, W. B. Guggino, W. A. Hendrickson, J. F. Hunt, M. C. Kearins, D. Lorimer, P. C. Maloney, K. W. Post, K. R. Rajashankar, M. E. Rutter, J. M. Sauder, S. Shriver, P. H. Thibodeau, P. J. Thomas, M. Zhang, X. Zhao, and S. Emtage. 2004. Structure of nucleotide-binding domain 1 of the cystic fibrosis transmembrane conductance regulator. EMBO J. 23:282-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loo, M. A., T. J. Jensen, L. Cui, Y. Hou, X. B. Chang, and J. R. Riordan. 1998. Perturbation of Hsp90 interaction with nascent CFTR prevents its maturation and accelerates its degradation by the proteasome. EMBO J. 17:6879-6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mancini, R., M. Aebi, and A. Helenius. 2003. Multiple endoplasmic reticulum-associated pathways degrade mutant yeast carboxypeptidase Y in mammalian cells. J. Biol. Chem. 278:46895-46905. [DOI] [PubMed] [Google Scholar]
- 28.Marcus, N. Y., and D. H. Perlmutter. 2000. Glucosidase and mannosidase inhibitors mediate increased secretion of mutant alpha1 antitrypsin Z. J. Biol. Chem. 275:1987-1992. [DOI] [PubMed] [Google Scholar]
- 29.McClellan, A. J., and J. Frydman. 2001. Molecular chaperones and the art of recognizing a lost cause. Nat. Cell Biol. 3:E51-E53. [DOI] [PubMed] [Google Scholar]
- 30.Meacham, G. C., Z. Lu, S. King, E. Sorscher, A. Tousson, and D. M. Cyr. 1999. The Hdj-2/Hsc70 chaperone pair facilitates early steps in CFTR biogenesis. EMBO J. 18:1492-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meacham, G. C., C. Patterson, W. Zhang, J. M. Younger, and D. M. Cyr. 2001. The Hsc70 co-chaperone CHIP targets immature CFTR for proteasomal degradation. Nat. Cell Biol. 3:100-105. [DOI] [PubMed] [Google Scholar]
- 32.Molinari, M., V. Calanca, C. Galli, P. Lucca, and P. Paganetti. 2003. Role of EDEM in the release of misfolded glycoproteins from the calnexin cycle. Science 299:1397-1400. [DOI] [PubMed] [Google Scholar]
- 33.Morimoto, R. I. 1998. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 12:3788-3796. [DOI] [PubMed] [Google Scholar]
- 34.Morris, A. P., S. A. Cunningham, D. J. Benos, and R. A. Frizzell. 1993. Glycosylation status of endogenous CFTR does not affect cAMP-stimulated Cl secretion in epithelial cells. Am. J. Physiol. 265:C688-C694. [DOI] [PubMed] [Google Scholar]
- 35.Oda, Y., N. Hosokawa, I. Wada, and K. Nagata. 2003. EDEM as an acceptor of terminally misfolded glycoproteins released from calnexin. Science 299:1394-1397. [DOI] [PubMed] [Google Scholar]
- 36.Okiyoneda, T., K. Harada, M. Takeya, K. Yamahira, I. Wada, T. Shuto, M. A. Suico, Y. Hashimoto, and H. Kai. 2004. Delta F508 CFTR pool in the endoplasmic reticulum is increased by calnexin overexpression. Mol. Biol. Cell 15:563-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parodi, A. J. 2000. Protein glucosylation and its role in protein folding. Annu. Rev. Biochem. 69:69-93. [DOI] [PubMed] [Google Scholar]
- 38.Peterson, J. R., A. Ora, P. N. Van, and A. Helenius. 1995. Transient, lectin-like association of calreticulin with folding intermediates of cellular and viral glycoproteins. Mol. Biol. Cell. 6:1173-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pind, S., J. R. Riordan, and D. B. Williams. 1994. Participation of the endoplasmic reticulum chaperone calnexin (p88, IP90) in the biogenesis of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 269:12784-12788. [PubMed] [Google Scholar]
- 40.Qu, B. H., and P. J. Thomas. 1996. Alteration of the cystic fibrosis transmembrane conductance regulator folding pathway. J. Biol. Chem. 271:7261-7264. [DOI] [PubMed] [Google Scholar]
- 41.Seibert, F. S., Y. Jia, C. J. Mathews, J. W. Hanrahan, J. R. Riordan, T. W. Loo, and D. M. Clarke. 1997. Disease-associated mutations in cytoplasmic loops 1 and 2 of cystic fibrosis transmembrane conductance regulator impede processing or opening of the channel. Biochemistry 36:11966-11974. [DOI] [PubMed] [Google Scholar]
- 42.Sharma, M., F. Pampinella, C. Nemes, M. Benharouga, J. So, K. Du, K. G. Bache, B. Papsin, N. Zerangue, H. Stenmark, and G. L. Lukacs. 2004. Misfolding diverts CFTR from recycling to degradation: quality control at early endosomes. J. Cell Biol. 164:923-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strickland, E., B. H. Qu, L. Millen, and P. J. Thomas. 1997. The molecular chaperone Hsc70 assists the in vitro folding of the N-terminal nucleotide-binding domain of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 272:25421-25424. [DOI] [PubMed] [Google Scholar]
- 44.Svedine, S., T. Wang, R. Halaban, and D. N. Hebert. 2004. Carbohydrates act as sorting determinants in ER-associated degradation of tyrosinase. J. Cell Sci. 117:2937-2949. [DOI] [PubMed] [Google Scholar]
- 45.Thibodeau, P. H., C. A. Brautigam, M. Machius, and P. J. Thomas. 2005. Side chain and backbone contributions of Phe508 to CFTR folding. Nat. Struct. Mol. Biol. 12:10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tokunaga, F., C. Brostrom, T. Koide, and P. Arvan. 2000. Endoplasmic reticulum (ER)-associated degradation of misfolded N-linked glycoproteins is suppressed upon inhibition of ER mannosidase I. J. Biol. Chem. 275:40757-40764. [DOI] [PubMed] [Google Scholar]
- 47.Tsai, B., Y. Ye, and T. A. Rapoport. 2002. Retro-translocation of proteins from the endoplasmic reticulum into the cytosol. Nat. Rev. Mol. Cell Biol. 3:246-255. [DOI] [PubMed] [Google Scholar]
- 48.Urban, S., and M. Freeman. 2003. Substrate specificity of rhomboid intramembrane proteases is governed by helix-breaking residues in the substrate transmembrane domain. Mol. Cell 11:1425-1434. [DOI] [PubMed] [Google Scholar]
- 49.Varga, K., A. Jurkuvenaite, J. Wakefield, J. S. Hong, J. S. Guimbellot, C. J. Venglarik, A. Niraj, M. Mazur, E. J. Sorscher, J. F. Collawn, and Z. Bebok. 2004. Efficient intracellular processing of the endogenous cystic fibrosis transmembrane conductance regulator in epithelial cell lines. J. Biol. Chem. 279:22578-22584. [DOI] [PubMed] [Google Scholar]
- 50.Vashist, S., and D. T. Ng. 2004. Misfolded proteins are sorted by a sequential checkpoint mechanism of ER quality control. J. Cell Biol. 165:41-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang, J., and A. L. White. 2000. Role of calnexin, calreticulin, and endoplasmic reticulum mannosidase I in apolipoprotein(a) intracellular targeting. Biochemistry 39:8993-9000. [DOI] [PubMed] [Google Scholar]
- 52.Wang, Y., and M. J. Androlewicz. 2000. Oligosaccharide trimming plays a role in the endoplasmic reticulum-associated degradation of tyrosinase. Biochem. Biophys. Res. Commun. 271:22-27. [DOI] [PubMed] [Google Scholar]
- 53.Ward, C. L., and R. R. Kopito. 1994. Intracellular turnover of cystic fibrosis transmembrane conductance regulator. Inefficient processing and rapid degradation of wild-type and mutant proteins. J. Biol. Chem. 269:25710-25718. [PubMed] [Google Scholar]
- 54.Ward, C. L., S. Omura, and R. R. Kopito. 1995. Degradation of CFTR by the ubiquitin-proteasome pathway. Cell 83:121-127. [DOI] [PubMed] [Google Scholar]
- 55.Wu, B. J., H. C. Hurst, N. C. Jones, and R. I. Morimoto. 1986. The E1A 13S product of adenovirus 5 activates transcription of the cellular human HSP70 gene. Mol. Cell. Biol. 6:2994-2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang, Y., S. Janich, J. A. Cohn, and J. M. Wilson. 1993. The common variant of cystic fibrosis transmembrane conductance regulator is recognized by hsp70 and degraded in a pre-Golgi nonlysosomal compartment. Proc. Natl. Acad. Sci. USA 90:9480-9484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Younger, J. M., H. Y. Ren, L. Chen, C. Y. Fan, A. Fields, C. Patterson, and D. M. Cyr. 2004. A foldable CFTR{Delta}F508 biogenic intermediate accumulates upon inhibition of the Hsc70-CHIP E3 ubiquitin ligase. J. Cell Biol. 167:1075-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang, F., N. Kartner, and G. L. Lukacs. 1998. Limited proteolysis as a probe for arrested conformational maturation of delta F508 CFTR. Nat. Struct. Biol. 5:180-183. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, H., K. W. Peters, F. Sun, C. R. Marino, J. Lang, R. D. Burgoyne, and R. A. Frizzell. 2002. Cysteine string protein interacts with and modulates the maturation of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 277:28948-28958. [DOI] [PubMed] [Google Scholar]
- 60.Zhang, Y., G. Nijbroek, M. L. Sullivan, A. A. McCracken, S. C. Watkins, S. Michaelis, and J. L. Brodsky. 2001. Hsp70 molecular chaperone facilitates endoplasmic reticulum-associated protein degradation of cystic fibrosis transmembrane conductance regulator in yeast. Mol. Biol. Cell 12:1303-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]