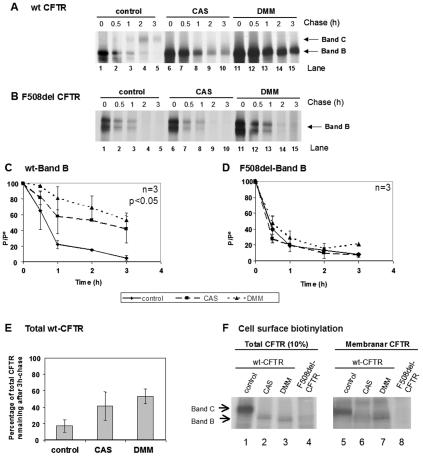
FIG. 5.
Turnover of wt and F508del-CFTR under CAS and DMM. CHO cells stably expressing (A) wt CFTR or (B) F508del-CFTR were treated with 1 mM CAS (lanes 6 to 10) or 1 mM DMM (lanes 11 to 15) or untreated (lanes 1 to 5). After a 90-min treatment, the cells were pulse-labeled and chased for 0 h (lanes 1, 6, and 11), 0.5 h (lanes 2, 7, and 12), 1 h (lanes 3, 8, and 13), 2 h (lanes 4, 9, and 14), and 3 h (lanes 5, 10, and 15). The cells were then lysed, immunoprecipitated with an anti-CFTR Ab, and analyzed as before (see the legend to Fig. 2) to determine the turnover of immature wt CFTR (C) and F508del-CFTR (D). (E) Percentage of total CFTR (band B plus band C) remaining at the end of the chase period in untreated cells compared with total band B in cells treated with either CAS or DMM. The number of experiments and statistically significant differences are indicated as in Fig. 2. (F) Cell surface biotinylation followed by CFTR IP was performed to determine whether protein produced under CAS or DMM reaches the cell surface. CHO cells expressing wt or F508del-CFTR were incubated for 72 h with CAS or DMM. Cell surface proteins were biotinylated, and CFTR IP was carried out. Samples were divided into two equal portions, and biotin-labeled proteins were captured with streptavidin beads in one of the fractions. Total and biotinylated (membrane) CFTRs were then in vitro phosphorylated using [γ-32P]ATP and cAMP-dependent protein kinase. Ten percent of total immunoprecipitated CFTR (lanes 1 to 4) and all immunoprecipitated membrane CFTR (lanes 5 to 8) were subjected to SDS-PAGE and fluorography. Band B of wt CFTR produced under CAS or DMM could be clearly detected in the membrane fraction.