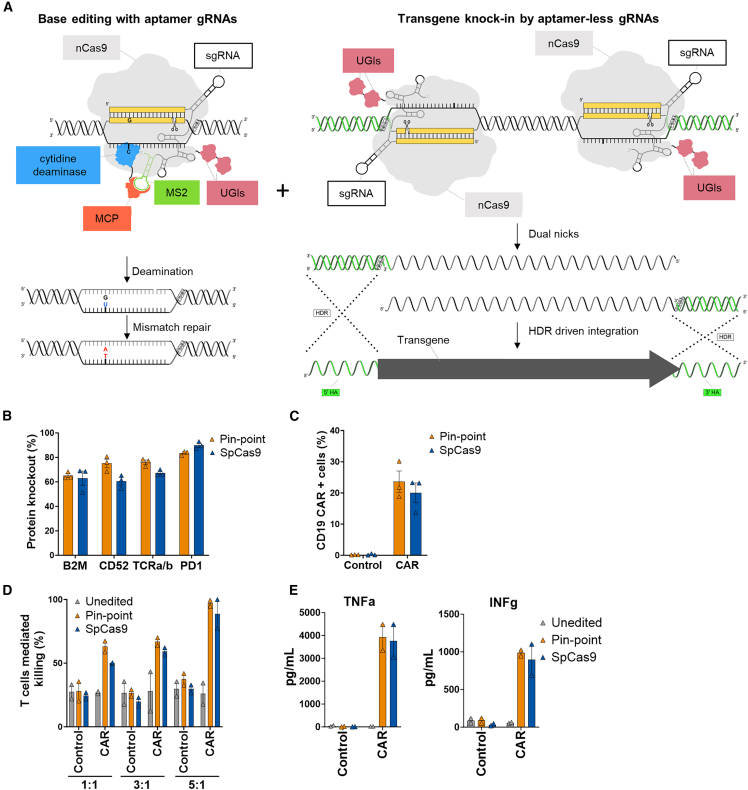
Figure 7.
Generation of multiplex edited CAR-T cells by simultaneous multiplex base editing knockout and locus-specific knockin with the Pin-point system
(A) Schematic showing the recruitment of the entire Pin-point platform machinery by aptamer-containing gRNAs on the site where the desired outcome is base editing (left) and of the nCas9 alone by aptamer-less gRNAs on the knockin site (right). CAR-T cells were generated by knockin of the CD19-CAR in the TRAC locus. Pin-point mRNAs have been co-delivered with aptamer-containing sgRNAs directed to base edit B2M, CD52, and PDCD1 and 2 aptamer-less sgRNAs designed to target the exon1 of TRAC locus. Cells electroporated with SpCas9 mRNA received optimal gRNAs to knock out B2M, CD52, and PDCD1 by indel formation and one of the two gRNA designed to target the exon 1 of TRAC locus. Shortly after electroporation, cells were transduced with AAV6 carrying the CD19-CAR transgene flanked by the homology arms to the TRAC locus. (B) Frequency of CD52, TCRa/b, PD1, and B2M protein knockout following co-delivery of Pin-point or SpCas9 reagents and transduction with the AAV6-CAR as analyzed by flow cytometry 7 days post electroporation/transduction. (C) Frequency of CD19-CAR-positive cells in the T cell population after delivery of either Pin-point or SpCas9 reagents and transduction with the AAV6-CAR compared with non-transduced cells. (D) Raji cell killing measured by calcein assay after co-culture with T cells unedited or multi-edited with the Pin-point platform or with SpCas9 and transduced with AAV6-CAR compared with non-transduced cells at 1:1, 3:1, or 5:1 T cell:target cell ratios. Control cells are non-transduced cells. (E) Levels of TNF alpha and INF gamma measured in the media of the co-culture at the 1:1 T cell:target cell ratio. Data represented as mean ± SD, n = 2 independent biological T cell donors.