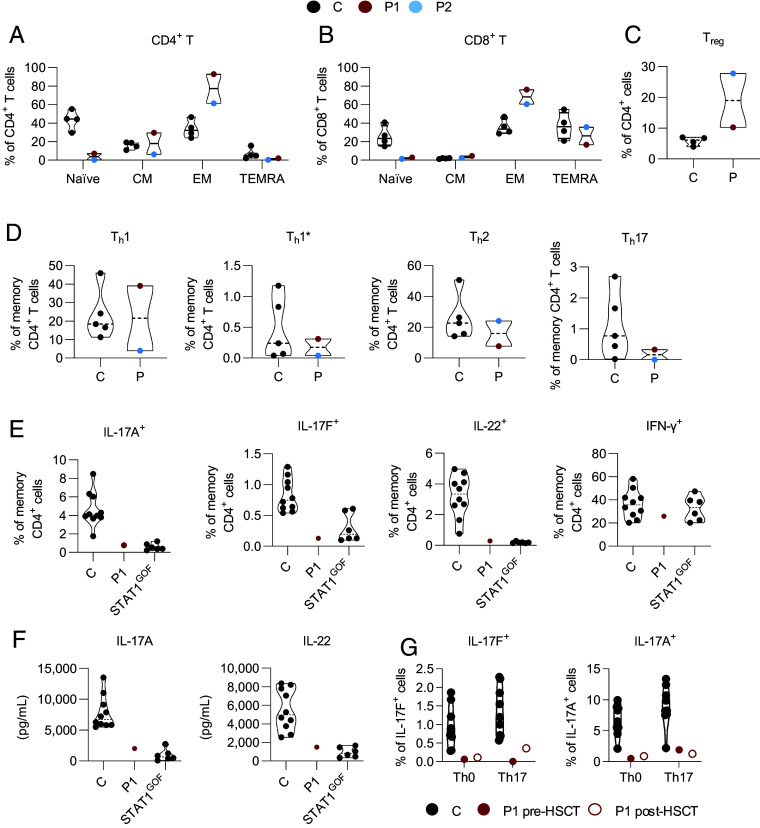
Fig. 6.
RelB deficiency compromises T cell development and functions. Immunophenotyping of PBMCs from 4-13 healthy controls (C, black dots), P1 (red dots), and P2 (blue dots). (A–C) Frequency of naïve (CD45RA+CCR7+), central memory (CM, CD45RA−CCR7+), effector memory (EM, CD45RA+/−CCR7−), and terminally differentiated effector memory (TEMRA, CDR45RA−CCR7−) cells among the CD4+ (A) and CD8+ (B) T cells of healthy subjects (C, n = 4), P1 before HSCT, and P2. (C) Frequency of Treg (CD3+CD4+CD25hiFoxP3+) cells in the CD4+ T cell compartment in controls (n = 4), P1 before HSCT, and P2. (D) Frequencies of Th subsets among memory CD4+ T cells from controls (n = 5), P1 before HSCT, and P2. Subsets were defined as follows: Th1 (CXCR5−CXCR3+CCR4−CCR6−), Th1* (CXCR5−CXCR3+CCR4−CCR6+), Th2 (CXCR5−CXCR3−CCR4+CCR6−), and Th17 (CXCR5−CXCR3−CCR4+CCR6+). Dotted horizontal lines represent the median value in each distribution. (E) Percentages of cells expressing IL-17A, IL-17F, IL-22, and IFN-γ ex vivo, as determined by flow cytometry, among memory CD4+ T cells from PBMCs activated by incubation with PMA and ionomycin for 8 h. (F) IL-17A and IL-22 production assessed by specific ELISA on whole-blood supernatants stimulated by incubation for 24 h with PMA and ionomycin. The two experiments were conducted in parallel for healthy subjects (n = 10), the RelB-deficient patient (P1), and patients with heterozygous gain-of-function STAT1 mutations (n = 6). (G) Cytokine production, measured by ELISA, for IL-17A and IL-17F after 4 d of culture in Th0 or Th17 conditions, for cells from nine different healthy donors, and from P1 before (pre-) and after (post-) HSCT.