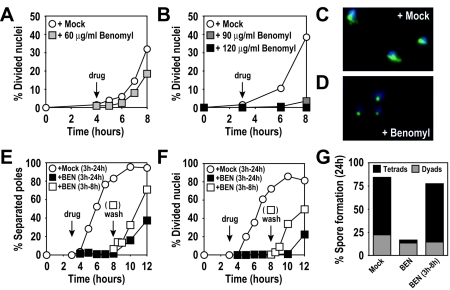
FIG. 1.
High levels of benomyl reversibly arrest meiotic cells. (A) Wild-type (A727) cells. At 4 h after meiotic induction at room temperature (black arrow with the label “drug”), cells were resuspended in medium containing 60 μg/ml benomyl (grey squares) or 0.4% DMSO (mock; white circles). (B) Wild-type (A727) cells. At 3 h after meiotic induction at 30°C (black arrow), cells were resuspended in medium containing 90 μg/ml benomyl (dark grey squares), 120 μg/ml benomyl (black squares), or 0.4% DMSO (mock, white circles). The percentages of cells in the results shown in panels A and B that underwent at least one nuclear division were determined by DAPI (4′,6-diamidino-2-phenylindole) staining at the indicated time points. (C and D) Wild-type (A727) cells from cultures treated with 0.4% DMSO (C) or 120 μg/ml benomyl (D) fixed 1 h after treatment. DAPI-stained nuclear masses are shown in blue, and tubulin is shown in green. (E to G) Wild-type (A727) cells. At 3 h after meiotic induction at 30°C (arrow with the label “drug”), cells were resuspended in medium containing 120 μg/ml benomyl (black squares) or 0.4% DMSO (mock; white circles). At 5 h after drug addition (arrow labeled “wash”), the benomyl culture was split, and half the culture was washed and released into fresh sporulation medium containing 0.4% DMSO (white squares). (E) Percentage of cells containing more than one focus of tubulin staining. (F) Percentage of cells having undergone nuclear divisions (DAPI). (G) Spore formation 24 h after induction of meiosis. Asci were classified as containing two spores (dyads) or three or four spores (tetrads) (500 cells were analyzed for each condition).