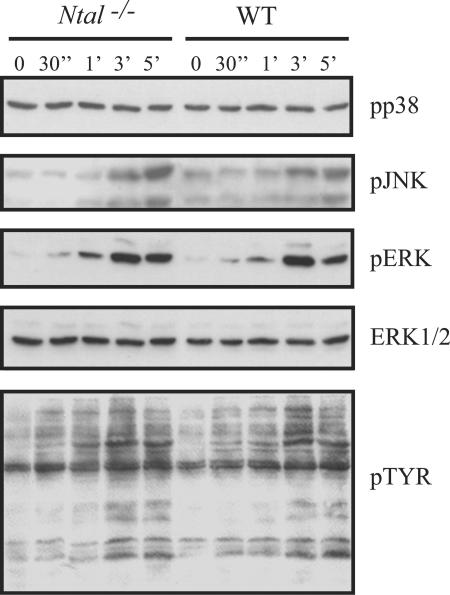
FIG. 6.
Analysis of protein tyrosine phosphorylation and of mitogen-activated protein kinase phosphorylation in splenic B cells from Ntal−/− mice. Purified B cells of Ntal−/− (lanes 1-5) and wild-type mice (lanes 6-10) were either left untreated or activated with affinity purified F(ab)′2 fragments of an anti-IgM monoclonal antibody for the indicated periods of time. Postnuclear lysates were subsequently separated by 12% SDS-PAGE and the activation of the individual members of the mitogen-activated protein kinase family (ERK, JNK, p38) assessed using phosphospecific anti-JNK, anti-p38, and anti-ERK antibodies. For analysis of global tyrosine phosphorylation, the antiphosphotyrosine monoclonal 4G10 antibody was employed. To verify equal loading, the blots were stripped and reincubated with an anti-ERK1/2 polyclonal rabbit antiserum. The data shown are representative of seven independently performed experiments.