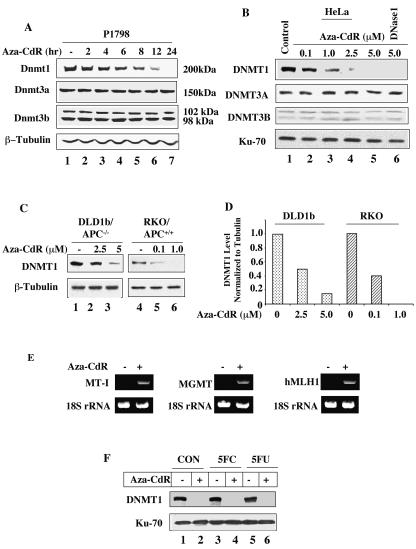
FIG. 1.
DNMT1 is selectively degraded by 5-aza-CdR treatment of mammalian cells. (A) Dnmt1, Dnmt3a, and Dnmt3b protein levels in P1798 cell extracts. Western blot analysis of Dnmt1/3a/3b with WCEs from mouse lymphosarcoma cells (100 μg of protein) treated with 5-aza-CdR (2.5 μM) for various times with antibodies specific for these proteins. The blots were reprobed with β-tubulin to show equal loading of proteins. (B) DNMT1, DNMT3A, and DNMT3B protein levels in extracts from HeLa cells treated with various concentrations of 5-aza-CdR. Western blot analysis with WCEs (250 μg of protein) from HeLa cells. The smaller (70-kDa) subunit of Ku antigen was used as the control to determine equal loading of proteins. (C and D) The 5-aza-CdR concentration required to deplete DNMT1 is proportional to the endogenous DNMT1 level. Immunoblot analysis of DNMT1 with WCEs (200 μg of protein) from wild-type and adenomatous polyposis coli (APC)-mutated colon cancer cells treated with various concentrations of 5-aza-CdR. The blots were reprobed with β-tubulin to demonstrate comparable levels of proteins in the lanes. For quantitation, the levels in untreated cells were taken as 1. (E) Activation of MT-I, human MLH1, and MGMT genes after 5-aza-CdR treatment. Total RNA was isolated from P1798 cells treated with 5 μM 5-aza-CdR for 24 h. The expression of MT-I, human MLH1, and MGMT mRNAs was analyzed by RT-PCR. 18S RNA was amplified from all of the samples to normalize RNA input. (F) HeLa cells were either left untreated or treated with 10 μM flucytosine (5FC) or 5-fluorodeoxyuridine (5FU) for 12 h followed by treatment with 5-azaC-dR (5 μM) for an additional 12 h. Extracts were subjected to Western blot analysis.