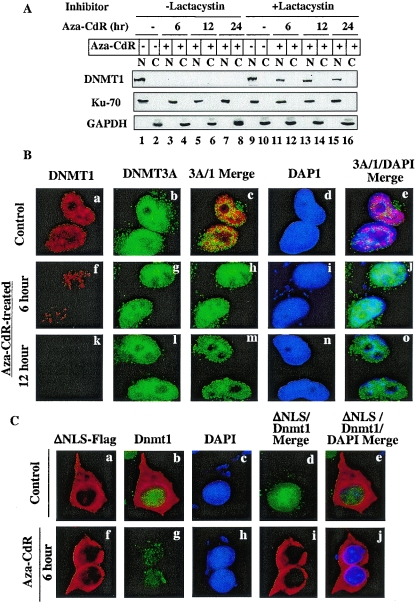
FIG. 4.
5-Aza-CdR-induced degradation of DNMT1 occurs in the nucleus and can be blocked by a proteasomal inhibitor (lactacystin). (A) Western blot analysis of DNMT1 in nuclear and cytoplasmic fractions. Cells were treated with 20 μM lactacystin for 1 h prior to exposure to 5-aza-CdR (5 μM). Nuclear (100 μg of protein) and cytoplasmic (100 μg of protein) extracts from these cells were subjected to Western blot analysis with anti-DNMT1, anti-Ku70, and anti-GAPDH antibodies. (B) Immunofluorescence staining of DNMT1 and DNMT3A in HeLa cells. Cells grown on coverslips were either left untreated or treated with 5-aza-CdR (5 μM) for various times. The fixed cells were stained with mouse anti-DNMT1 monoclonal antibodies (Imgenex) and rabbit anti-DNMT3A polyclonal antibodies followed by TRITC-conjugated anti-mouse immunoglobulin G (IgG) (for DNMT1) and FITC-conjugated anti-rabbit IgG (for DNMT3A). Nuclei were stained with 4′,6′-diamidino-2-phenylindole (DAPI) and visualized under a fluorescence microscope (Nikon Eclipse 800). (C) Endogenous nuclear Dnmt1 (Cos-7) but not cytoplasmic ectopic mouse Dnmt1 is sensitive to 5-aza-CdR-induced degradation. Cos-7 cells expressing nuclear localization signal-deleted Dnmt1-Flag (ΔNLS) were stained with rabbit anti-DNMT1 antibody (for endogenous enzyme) and mouse anti-Flag monoclonal antibody M2 (for ectopic Dnmt1). FITC-conjugated anti-rabbit IgG and TRITC-conjugated anti-mouse IgG were used to detect the respective proteins.