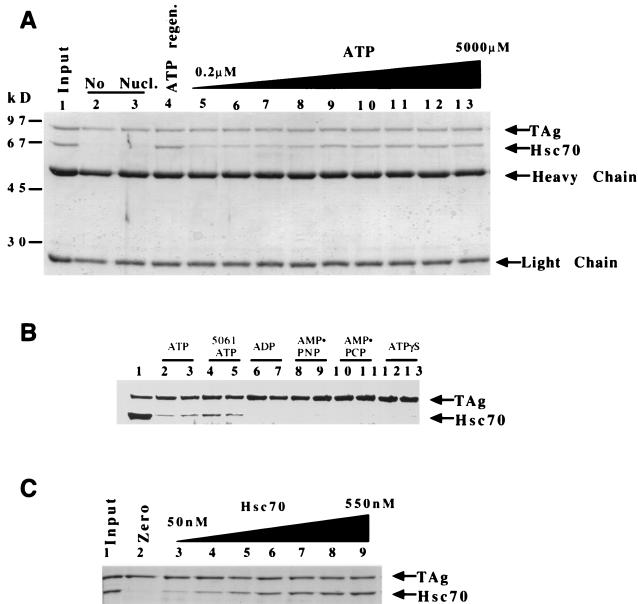
FIG. 3.
Hsc70 binding to T antigen requires ATP hydrolysis and is concentration dependent. (A) T antigen (120 nM) and Hsc70 (300 nM) were incubated and then immunoprecipitated for T antigen. A Coomassie blue-stained gel shows associated proteins. Results for 100% input of anti-T-antigen PAb416 and 50% input of T antigen and Hsc70 are shown (lane 1). Reactions containing no ATP (lanes 2 and 3), an ATP regeneration system (1 mM) (lane 4), or increasing amounts of ATP (0.2 to 5,000 μM) are shown in order (lanes 5 to 13) 0.2, 0.5, 1.0, 5.0, 50, 100, 500, 1,000, 5,000 (μM), respectively. Heavy chain and light chain refer to the antibody that coprecipitates in the immunoprecipitation reaction. (B) Two concentrations (1 or 4 mM) of ATP (lanes 2 and 3), ADP (lanes 6 and 7), AMP-PNP (lanes 8 and 9), AMP-PCP (lanes 10 and 11), and ATPγS (lanes 12 and 13) were incubated with T antigen and Hsc70, and the reactions were immunoprecipitated for T antigen with PAb416 as described in Materials and Methods. Lanes 4 and 5 show the reaction with 5061, a T-antigen ATP binding mutant, incubated with ATP (1 and 4 mM, respectively). (C) Hsc70 concentration dependence. T antigen (120 nM) was incubated with Hsc70 (0 to 550 nM). Lane 1, 50% input of T antigen and 1 μg of Hsc70 protein; lanes 2 to 9; Hsc70 at 0, 50, 110, 230, 290, 340, 400, and 550 nM, respectively.