Abstract
Background
Africa is experiencing a significant surge in the use of pesticides on farms. Though the use of pesticide products on farms is increasing rapidly, the ability to monitor and regulate the practice has not kept pace. Despite their potential significance, the health and environmental impacts of the growing pesticide usage in developing nations remain inadequately comprehended and recorded.
Objective
This paper presents a research protocol for a study that seeks to provide criteria for future monitoring of pesticide residues in aquatic environments and food sources. This study aims to evaluate pesticide utilisation methods and the potential hazards of pesticide residues in aquatic ecosystems. Additionally, the study seeks to assess the human health risks linked to pesticide applications.
Methods
This study will employ a quantitative approach and cross-sectional design. It will utilise a combination of survey and the collection of biological and environmental samples. Our methodology consists of four distinct steps. These outline the processes for studying pesticide residue in environmental and fish samples. Additionally, we plan to employ mathematical algorithms to evaluate the ecological and health risks associated with these pesticide residues.
Conclusion
This study is an effort to monitor and assess the hazards to the environment and human well-being associated with the increasing utilisation of pesticides. It also aims to gather relevant data on pesticide utilisation practices that contribute to the contamination of aquatic ecosystems. It will specifically focus on determining the concentration of pesticide residues in both biological and environmental samples. Additionally, the study will assess the ecological and health risks associated with these pesticide residues. This will enable the incorporation of organised research efforts and coordinated pesticide surveillance operations for toxicovigilance.
Keywords: Pesticide monitoring, Risk assessment, Mixture toxicity, Fish muscle, Water, Sediment, Contamination, Gas chromatography
1. Introduction
The increasing utilisation of pesticides in African agriculture, along with a restricted ability to examine and oversee pesticide remnants, presents a significant challenge to the concept of one-health. Multiple studies have documented the occurrence of illicit pesticide marketing and substandard application of pesticide products in Africa [1,2]. International aid agencies typically consider pesticide residues in environmental and local food items to be a significant issue that requires intervention [3]. Pesticides have beneficial impacts on their targeted creatures. However, when pesticide residues infiltrate water bodies, they present significant risks to water resources and the ecosystems [4]. The challenge of implementing specific treatments arises from a lack of knowledge on whether the assumed residues surpass the maximum residue limits and their potential implications for ecological and health hazards. Pesticide regulation is essential at the national and regional levels in any country. It serves to govern the sale of pesticides and guarantees environmental preservation and consumer well-being. However, it is evident that many African nations experiencing an increased need for food have had a phase of swift expansion in the pesticide industry. This has resulted in over reliance on agricultural pesticides. High-income nations like the United States and European countries have the ability to actively monitor and track pesticide usage. In contrast, Sub-Saharan Africa (SSA) has low ability to monitor and track pesticide usage. The United States Department of Agriculture's (USDA) 'Interregional Research Project Number 4' (IR-4 Project), launched in 1963, has been generating data to determine pesticide tolerance and facilitate the safe registration and effective pest control measures with the Environmental Protection Agency (EPA) in the United States [5]. Some studies have shown widespread occurrence of pesticides in several ecosystems worldwide [[6], [7], [8], [9], [10]]. A prior research has demonstrated that excessive quantities of pesticides over the threshold limits can significantly pollute the environment, namely in water bodies and food sources, and lead to severe health complications [11]. Pesticides Organochlorines (OCPs) exert toxicity on reproductive, respiratory, immune systems and endocrine glands [12,13]. Moreover, they possess mutagenic and carcinogenic properties [14] and have the ability to impair the growth of aquatic organisms.
African countries, including Uganda, Ghana, and Nigeria, have conducted research to evaluate and record the levels of pesticide residues found in commonly consumed fruits and vegetables [[15], [16], [17], [18]]. However, limited focus has been given to environmental samples, particularly those pertaining to the aquatic environment (such as water reservoirs) and their products. This raises concerns about the environmental issues that have health implications in Ghana and beyond. As far as we know, no studies have been conducted in Ghana which have incorporated variables such as pesticide use practices and farmers’ awareness of aquatic ecosystems contamination. Previous studies have mostly examined farmers' perspectives, patterns of use, methods of handling and managing pesticides, and their assessment of the associated risks [[19], [20], [21]]. However, it has been proven that the inappropriate use of pesticides by farmers is a major cause of pesticide residue contamination in aquatic ecosystems [22]. Thus, it has become crucial to investigate the pesticide use practices of farmers that could potentially lead to contamination of aquatic ecosystems.
According to the Ministry of Food and Agriculture (MoFA), the agriculture industry is a significant national contributor to Gross Domestic Product (about 22.99 % in 2021) in Ghana [23]. This substantial contribution is achieved mainly through the utilisation of large volumes (about 42,609,102 L) of chemical pesticides, [23]. These are sourced from many origins and of varying quality. This leads to their discharge into aquatic ecosystems including rivers, dams, and other reservoirs. These serve as crucial habitats for diverse forms of life. Thus, the presence of this combination of pesticides poses a potential threat to the biodiversity. It increases the likelihood of biodiversity depletion. Additionally, the inland fish captures about 145.272 metric tonnes of the county's fisheries [23]. In 2017, these reservoirs accounted for almost 17 % of the fresh fish consumed in northern Ghana [24]. This elevates the likelihood of ingesting fish that is contaminated. Though there have been studies on pesticide residues in vegetables in some African countries, there is a lack of comprehensive information at the national and regional level on pesticide use practices that contribute to contamination of aquatic ecosystems. This poses a significant risk to aquatic biodiversity and human health, particularly through the consumption of fish from these water bodies. Therefore, this study protocol addresses the aforementioned knowledge gap and provides guidance for future capacity-building initiatives. The primary objective is to gain a comprehensive understanding of the specific characteristics, quantities, variations, and frequencies of pesticide residues present in aquatic environments and fish consumed in northern Ghana. This knowledge will support a more accurate assessment of the ecological and health risks associated with pesticide residues. Additionally, this study covers relevant matrices (water, sediment, and fish). It integrates contributors to the pesticide residue issue to its health implications. Typically, pesticide residue studies concentrate on singular matrices. Similarly, health-focused studies often target specific outcomes rather than assessing the full spectrum of potential health risks from pesticide contamination. By examining both ecosystems, our research will provide new data previously unavailable. This information will be used to guide decision-makers to implement effective outreach programs and enhance aquatic ecosystem management. Additionally, it will also serve as a reference for researchers to design future studies aimed at improving food and environment surveillance and reducing pesticide residues in Africa.
It is crucial to enhance our understanding of the environmental and health aspects of pesticide usage in agriculture. The findings from this study may support the implementation of evidence-based initiatives for current and future pesticide capacity programs and monitoring in Africa. Thus, the study protocol addresses the process of intervention research, ranging from agricultural practices to human health aspect of pesticide residue concern. This study protocol aims to address various aspects related to pesticide use in aquatic environments. It outlines the procedure for:
-
•
Assessing farmers' awareness and pesticide use practices associated with aquatic ecosystems contamination with pesticide residues,
-
•
Evaluating the levels of agricultural pesticide residues present in water, sediment, and fish within water reservoirs,
-
•
Determining the ecological risks posed by agricultural pesticide use in aquatic environments including potential impact on aquatic biodiversity and ecosystem health,
-
•
Investigating the potential human health risks associated with the consumption of fish from these ecosystems.
2. Methods
2.1. Study design and approach
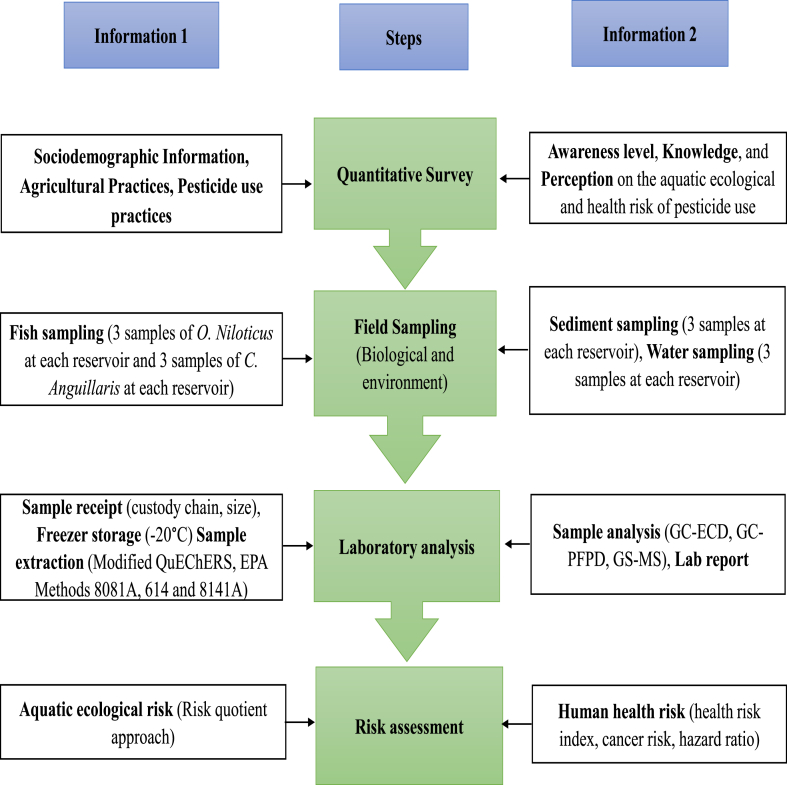
This study will employ a quantitative approach and cross-sectional design, involving 243 agricultural farm owners utilising pesticides in their farming practices between May 2024 and August 2024. The chosen period coincides with the rainy season, when there is increased pesticide usage. Consequently, it is anticipated that significant quantities of pesticide residue will be present. This study will employ a four-step approach (Fig. 1): i) administer a survey questionnaire to a specific group of farmers to evaluate the pesticide application practices that contribute to the contamination of water bodies with pesticide residues. ii) collect environmental samples (water and sediment) and biological samples (fish muscle) on site to determine the presence of pesticide residue. iii) analyse the collected samples in a laboratory to measure the quantity of pesticide residues present. iv) conduct a risk assessment to evaluate the potential harm to aquatic organisms and the health risks associated with consuming fish from these reservoirs. The process adheres to a quantitative methodology.
Fig. 1.
Flow diagram of the principal steps of pesticide residue evaluation. O = Oreochromis, C = Clarias, GC = Gas chromatography, ECD = Electron Capture Detector; PFPD = Pulsed Flame Photometric Detector; MS = Mass Spectrometry.
2.2. Study setting
The study will take place in the Libga and Builpela communities, which are located along the banks of the White Volta sub-basin catchment area in Northern Ghana. Approximately 80 % of the employment opportunities in these villages are connected to agriculture, namely conventional agricultural methods. Agriculture is the main industry in the region [17]. Previous studies have documented the presence of pesticide residues in various aquatic environments within the region, including reservoirs analogous to those examined in this study [25,26]. The Libga Reservoir and the Builpela Dam were chosen for the assessment of pesticides residue level, ecological danger, and health risk in the two communities. Knowing the broader context of agricultural practices and pesticide usage in the surrounding areas [2,27,28] suggests a notable risk of contamination. Considering the critical value of these water bodies for local communities, the potential impact of pesticide residues on their ecological health and safety was a key consideration in our study. The Libga Reservoir is situated in the Savelugu Municipality, at coordinates 9° 35′ 20″N and 0° 51′ 13″W. It is classified as a medium-sized reservoir, with a maximum area of 48 ha, according to Ref. [18]. The Builpela Dam, a compact reservoir, is located in the Builpela Community within the Tamale Metropolis, at coordinates 9° 23′ 00″N and 0° 50′ 22″W. The selection of these reservoirs was purposive and based on the following factors:
-
•
Reservoirs that have not undergone desilting after construction.
-
•
Inhabiting biodiversity: The Libga Reservoir exhibited greater values for all fish species diversity indices compared to the Bontanga Reservoir, which is the largest reservoir in the region. These indices include species richness (2.4 vs. 1.1), species diversity (2.4 vs. 1.6), and species evenness (0.52 vs. 0.40) [19].
-
•
Economically significant: The chosen reservoirs have the potential to increase freshwater fish production and management in the region. They currently support a major fishing industry that supplies fish for local consumption.
-
•
Proximity to agriculture fields: The minimal distance separating agricultural fields from the reservoirs may result in water contamination due to the presence of pesticide residues.
-
•
Water supply: The Libga Reservoir receives water primarily from rainfall and, during flood periods, from the White Volta River, which passes through the major agricultural communities in Northern Ghana. Precipitation that travels through the surrounding agricultural areas replenishes the Builpela Dam.
2.3. Data collection procedures
2.3.1. Agricultural practices and pesticide use assessment
Before conducting the survey, a preliminary meeting will be organised with the farmers and local officials. During this meeting, the methods and the benefits will be described to all parties involved.
-
•
Participants' eligibility and sampling process
The study will target all farmers utilising pesticides in any crop cultivation within the two chosen agricultural communities (Libga and Builpela). The selection will be based on the following criteria: (1) engaging in the activity at one of the two locations; and (2) engaging in the activity for a minimum of three years.
The selection of participants will be conducted through stratified sampling. The Ghana Statistical Services (GSS) will provide the total number and list of all farmers in each chosen community. The list will contain gender information, which will be compiled to create a sampling frame. The gender information will ensure an equitable opportunity for males and females to be chosen as participants in the study. A probability proportional to the size will be conducted to determine the number of participants that will be selected. To ensure thoroughness, a checklist (see supplementary materials) has been created, and each prospective participant will be assigned a distinct identifier. A random number generator will be used to select participants from the list of each site.
-
•
Sample size estimation
The sample size for this study will be calculated using formula (1) as follows: [29],
| n0 = Z2pq/e2 | (1) |
where n0 = desired sample size; Z = the normal standard deviation, set at α = 0.05, CI = 1.96; p = estimated proportion of farmers using pesticides for farming (17.5 % or 0.175); q = the acceptable deviation from the supposed proportion = (1−p); e = desired level of precision (5.0 %).
The sample size may be larger than if the true variability (P) of the population attribute was used. Thus, the estimated sample size is 221. As non-response and missing some participants can occur, 10 % of the estimated sample size will be added. The total sample size (n0) will be 243.
-
•
Instrument and data collection process
This study will use three weeks for the survey investigation. A meticulously designed questionnaire (see supplementary materials) will be created utilising the Kobo toolbox and implemented through in-person interviews using an Android tablet. A pretest of the questionnaire will be conducted with a sample of 20 farmers who will be randomly selected from the Builpela community in the Tamale metropolitan area. In Tamale Metropolis, farmers will be selected for the pre-test due to two main reasons. Firstly, Tamale is recognised as a significant hub for pesticide commerce in the northern area [2]. Secondly, farmers in this metropolis have convenient access to pesticides. The survey instrument will comprise a synchronised digital tablet and a backend database that can be accessed in Excel format for analysis. The question format will comprise a combination of closed-ended and partially categorised questions. Throughout the study, participants will be visited either at their workplaces on the farms or at their homes. The questions (Table 1) are designed to evaluate the agricultural and pesticide utilisation practices that contribute to the pollution of water bodies. This assessment helps to understand and analyse the connection between these practices and the contamination of water bodies. Moreover, this quantitative survey will provide a report on any potential hazards to aquatic organisms and humans that may arise from consuming aquafood sourced from these water bodies. The questionnaire will be formulated in the English language. However, to ensure precise data collection from participants who are unable to communicate or comprehend, research assistants will translate the questions into the local language of the participants (Dagbani). Three research assistants and the research team will be responsible for administering the questionnaire. For a research assistant to be recruited, they must meet the following criteria: hold a minimum of a bachelor's degree in environmental science, possess a proven track record of conducting quantitative research surveys in the field of environmental science, specifically focusing on studies related to pesticides, and demonstrate fluency in the main local language, Dagbani. Prior to data collection, the research assistants will undergo a three-day training programme encompassing both theoretical and practical aspects. The theory component will encompass subjects related to fundamental research principles, study purpose and objectives, data gathering, and interviewing methodologies. Exploration of the principles of informed consent and research ethics in studies involving human subjects. The fieldwork will involve hands-on activities and will be used to test the effectiveness of the data gathering instruments. Then, the instruments will be improved based on the data and feedback obtained from the research assistants.
Table 1.
Information that will be collected during the survey using a structured questionnaire.
| Questionnaire sections | Data to be collected |
|---|---|
| Sociodemographic and economic information | Age (years), sex, marital status (Current status), Education level (last grade completed), Main activity, secondary activities. Years of experience in farming |
| Agricultural practices | Crops cultivated (during the year); Farm size (acre), crops treated with pesticides, quantities of pesticide applied (liter), farm distance from the nearest water body, practice of irrigation, practice of off-season farming, spaying equipment washing practice, disposal of used pesticide containers (action engaged with used pesticide containers) |
| Pesticide use practices | Group of pesticide use during the last 12 months and last week, frequency of applications (in number of times), mode of application, disposal of leftover pesticide (action engaged with leftover pesticide solutions), practice of overuse and overdose of pesticides, pesticide application and management practices (action taken after pesticide use, pesticides storage, mixing of pesticides at the water reservoir) |
| Awareness level, knowledge, and perception of the aquatic ecological and health risks of pesticide use | Awareness of pesticide residues release in the environment, knowledge of the main receptacle of pesticide residue, awareness of the risks of water body's contamination, awareness of the harmful impacts of pesticides on fish and other water bodies' microorganisms, knowledge of the harmful effects of pesticides (type of effect), means of awareness of the harmful effect of pesticides, familiarity with regulations and guidelines for proper pesticide disposal and use, training attendance on pesticide handling and disposal, awareness of the harmful effects on human from consuming contaminated fish, health hazards (effects) to aquatic organisms and human health from eating contaminated fish. |
Prior to participation, participants will receive a comprehensive explanation of the study's objectives, methodology, duration, advantages, and potential hazards. Additionally, the measures will be taken to ensure privacy and confidentiality. To mitigate the possibility of revealing personal or sensitive data, individuals responsible for collecting data will get training to guarantee that participants refrain from sharing any personal or sensitive information that makes them feel uneasy. Furthermore, participants are under no obligation to answer any inquiries unless they feel at ease to do so. Participants will also be informed of their autonomy to terminate the discussion at any point if necessary. Furthermore, we will arrange appointments at participants' preferred times and make efforts to minimise waiting time, ensuring that interviews are conducted promptly. Study participants will be required to provide written informed consent before enrolment. Individuals without literacy skills will be asked to indicate their response by making an 'O' mark. The informed consent form will undergo translation into the local language, specifically Dagbani. This study will not include minors and thus, an assent form will not be required. After data collection, all files will be maintained on a secured computer. The primary investigator will be responsible for maintaining the database. De-identified data will be utilised for all analyses.
2.3.2. Contamination level assessment of pesticide residues
To evaluate the level of contamination in the reservoirs, we will gather biological samples (specifically, flesh from fish) and environmental samples (water and sediment) from the chosen reservoirs. This collection will take place over the same time period, as outlined in the study design, which coincides with the agricultural rainy season.
-
•
Biological Material
This study will utilise two fish species, namely Oreochromis (O.) niloticus and Clarias (C.) anguillaris. O. niloticus and C. anguillaris are often caught in northern Ghana, namely in the Libga and Builpela reservoirs [30]. Furthermore, these Fish species are found at distinct trophic levels and exhibit varying eating behaviours. They constitute a crucial element of the food patterns of the communities in the North [31]. O. niloticus, a fish that lives in open waters (pelagic fish) and is exposed to water column contaminants, mostly feeds on plants. This characteristic makes it an ideal subject for studying the effects of pollutants on the environment, particularly those chemicals that do not accumulate in the food chain. C. anguillaris, a water-dwelling species that primarily consumes both plants and fish (omnivorous with a piscivorous tendency), is an ideal choice for investigating the impact of persistent contaminants along the entire food chain. C. anguillaris is more susceptible to enduring harmful chemicals when exposed to fish in the water column, sediment, and lipophilic pesticide residues [32].
-
•
Biological and environmental sample collection
2.3.2.1. Fish muscle sample collection
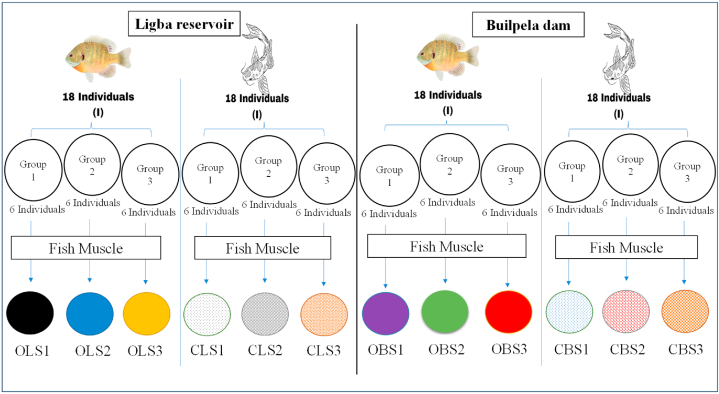
Prior to collecting the muscle sample, the initial procedure involves labelling the stomacher bags that will be used to retain the sample. The fish will be labelled using the initial letters of their names (O for Oreochromis, C for Clarias) and the reservoir names (L for Libga and B for Builpela), followed by S1, S2, and S1 to denote the sample numbers. The second stage (Fig. 2) entails the collection of the muscle sample. A total of 18 individuals of O. niloticus and 18 individuals of C. anguillaris, captured directly from each reservoir, will be sampled. Initially, at the Libga reservoir, a total of 18 individuals of O. niloticus will be divided into three distinct pools or groups. Each pool will consist of six individuals that are randomly picked. Prior to gathering the tissue, each specimen will be euthanized using a concentration of 100 mg/l of tricaine methanesulphonate (MS 222), as advised in the standard of the Organisation for Economic Co-operation and Development (OECD) for evaluating chemical substances [33]. The weight of each specimen will be measured using an Electronic Hanging Weight Scale (Fishfun® 110 lb/50 kg). Next, the dorsal muscles from each side of the fish in the first pool will be taken using a scalpel. These samples from the six individuals will be combined to create a single pooled sample weighing 1 kg. The sample will then be stored in a properly labelled stomacher bag lateral filter (OLS1) for the first pool. Next, the identical procedure will be employed to acquire two more muscle samples (each weighing 1 kg) of O. niloticus from the second and third groups. These samples will be collected in a stomacher bag lateral filter (OLS2 and OLS3) for the purpose of determining multi-pesticide residue levels.
Fig. 2.
Fish flesh (muscle) sample collection process at Libga and Builpela reservoirs. OLS1=Oreochromis Libga Sample 1, OLS2 = Oreochromis Libga Sample 2, and OLS3 = Oreochromis Libga Sample 3. CLS1 = Clarias Libga Sample 1, CLS2 = Clarias Libga Sample 2, and CLS3 = Clarias Libga Sample 3. OBS1 = Oreochromis Builpela Sample 1, OBS2 = Oreochromis Builpela Sample 2, and OBS3 = Oreochromis Builpela Sample 3. CBS1 = Clarias Builpela Sample 1, CBS2 = Clarias Builpela Sample 2, and CBS3 = Clarias Builpela Sample 3.
Similarly, the procedure employed for O. niloticus at the libga reservoir will be replicated in the group of 18 individuals of C. anguillaris to acquire three distinct samples of muscle (each weighing 1 kg), labelled CLS1, CLS2, and CLS3 [34]. have suggested this approach to assure representativeness and gather data that is significant and relevant.
The complete procedure employed in the Libga reservoir will be replicated at Builpela Dam to acquire three distinct muscle samples (each weighing 1 kg) of O. niloticus labelled OBS1, OBS2, and OBS3 (O = Oreochromis, B = Builpela, S = Sample), as well as three distinct muscle samples (each weighing 1 kg) of C. anguillaris labelled CBS1, CBS2, and CBS3. Every sample will be wrapped in aluminium foil, stored in a cooler ice box at a temperature of 4 ± 2 °C while in the field, and thereafter conveyed to the laboratory for preservation at −20 °C prior to analysis. The anticipated weight of the fish to be sampled is 300 g for C. anguillaris and 250 g for O. niloticus. These estimations are based on the presumption that at these weights, these species are prone to accumulating elevated quantities of pesticide residues in their tissues over a period of time. This can offer a precise depiction of the possible hazards associated with consuming fish that may harbour pesticide residues. A checklist (see supplementary materials) for fish muscle collection has been developed as an extra data collection tool to ensure proper adherence to all phases and procedures.
2.3.2.2. Sediment collection
The sediment collection will employ the conventional surface grab technique utilising a stainless-steel spoon. Three sediment samples (triplicate) weighing 1 kg each will be collected from each reservoir. The samples will be collected in polyethylene bags and will be one foot deep. At each site, a sample will be collected consisting of three equal 335 g samples taken at three sampling points in the reservoir. The sampling points will be spaced 10 feet apart, starting from downstream and moving upstream. Subsequently, all grab samples will be deposited in a sanitised plastic basin and meticulously homogenised using a suitable utensil. Continuous mixing prevents the stratification of the sample [35]. Prior to being placed in the collection basin, every effort will be made to eliminate any stones, roots, debris, foreign objects, and shells from the sample. Additionally, water will be carefully poured out of each sample grab. To obtain the final sample, the mixture will be combined, and then 1 kg of the mixture will be measured using a Fishfun® 110 lb/50 kg Electronic Hanging Weight Scale. The measured quantity will then be placed into suitable polyethylene bags. Upon completion of the collection process, the sample will be securely wrapped in aluminium foil, appropriately labelled, and stored in a cooler ice box at a temperature of 4 ± 2 °C in the field. The samples will be labelled using the initial letter of the reservoir's name (L for Libga and B for Builpela), followed by the initial letter of the sample type (S for sediment), and the sample number indicated by S1, S2, and S3. The aforementioned procedure will be duplicated for the remaining two composite samples.
The study will assign the labels LSS1, LSS2, and LSS3 to the Libga reservoir and BSS1, BSS2, and BSS3 to the Builpela Dam. According to Ref. [35], this method is cost-effective, non-mechanical, readily accessible, highly portable, user-friendly, and enables sampling of almost all types of sediment. The use of composite sampling has been deemed adequate for obtaining a representative sample from the sediment area being evaluated [36,37]. A supplementary data collection instrument (checklist) (see supplementary materials) has been developed to guarantee proper adherence to all steps and protocols during the process of sediment sampling.
2.3.2.3. Water sample collection
Following the collection of sediment, three water samples (each measuring 1 L) will be promptly collected from each reservoir at the identical location as for sediment collection. Each sample will consist of three subsamples, each measuring 335 ml, collected at three different points in the reservoir. These sampling points will be spaced 10 feet apart. The three subsamples will then be combined to form a pooled sample of 1 L, which will be collected in a certified plastic bottle. The subsamples will be obtained by immersing the sampling bottles at a depth of less than 0.3 m below the water surface, ensuring that they are completely filled without any air space in the bottleneck. Prior to labelling, the samples will be wrapped in aluminium foil. The labelling will consist of using the first letter of the reservoir's name (L for Libga and B for Builpela), the first letter of the sample type (W for Water), and S1, S2, and S3 to indicate the sample number. The labels assigned to the Libga reservoir are LWS1, LWS2, and LW3, while the Builpela Dam is labelled BWS1, BWS2, and BWS3. The samples will be stored in a cooler ice box at a temperature of 4 ± 2 °C in the field prior to being sent for pesticide analysis. The utilisation of various subsamples and multiple pooled samples enables the characterization of a diverse spectrum of chemical concentrations present in the reservoir. This approach ensures the provision of precise information and representative data [38]. An additional tool, in the form of a water sampling checklist (see supplementary materials), has been created to ensure that all steps and procedures are executed correctly.
2.3.2.4. Sample preparation
Before analysing samples for pesticide residues, the water sample will undergo filtration using 0.45 μm fibre glass filters (manufactured by Whatman) to eliminate any suspended particles. The sediment samples will undergo the process of air drying and subsequent sieving using a 250 μm stainless steel mechanical shaker. The fish samples will undergo a process of thawing and cleaning using distilled water. The muscle tissues will be finely chopped and then blended together.
2.3.2.5. Pesticide residue analysis
The dorsal muscle, sediment, and water samples will be stored in chilled cooler boxes at a temperature of 4 ± 2 °C and transported to the Pesticide Residue Laboratory of the Ghana Standards Authority Board. This laboratory is certified and specialises in conducting quantitative multi-residue pesticide analysis (https://www.gsa.gov.gh/). The extraction of pesticide residues from water samples will be conducted using US EPA Method 3510 [39], which is specifically designed for the analysis of semi-volatile and non-volatile organics in aqueous matrices. The extraction of pesticide residues from sediment and fish flesh samples will be performed using EPA Methods 8081A, 614, and 8141A, as well as the Modified QuEChERS method. Additionally, the choice of test for pesticide extraction will depend on the specific type of pesticide, with options including Gas Chromatography (GC) with an Electron Capture Detector (ECD), Pulsed Flame Photometric Detector (PFPD), or Gas Chromatography Mass Spectrometry (GC-MS). This study will focus on the detection and analysis of pesticides within the organophosphate and organochlorine groups without targeting any specific compound residues.
2.3.2.6. Method validation
The validation of the method will involve implementing stringent quality control procedures by examining solvent and procedure blanks. All reagents used will be of analytical grade. Samples will be analysed in triplicate, and mean concentrations will be estimated based on the number of samples yielding positive results to confirm the precision of the method. Specificity, this will be assessed by ensuring that the method distinguishes the target pesticides from other compounds, while sensitivity will be evaluated by determining the lowest concentration that can be reliably measured through limit of detection (LOD). Recalibration curves will be obtained for each batch of samples to ensure that the correlation coefficients are consistently maintained at a minimum value of 0.98. The limits of quantification (LOQ) for pesticides will be established for sediment and water. Assurance analysis will be conducted to verify that the pesticide determinations are accurate, sensitive, specific, and fall within acceptable values.
2.3.3. Aquatic ecological risk assessment of the reservoirs
The risk quotient (RQ) approach outlined in the "Technical Guidance Document on Risk Assessment from the European Commission" will be employed to evaluate the level of ecological hazards associated with pesticide usage. This approach adheres to mixture toxicity by incorporating the individual effects of each pesticide separately [40]. The value of RQ will be calculated using the following equation (2) [41]:
| RQ = MEC/PNEC | (2) |
PNEC is the Predicted No-Effect Concentration’’ and MEC is the Measured Environmental Concentration’’ of a pesticide in water. The MEC computation will only employ data when the pesticide concentrations are measured. The PNEC will be determined for both reservoirs using the following equation (3) [42]:
| PNEC = CC/AF | (3) |
CC is the Critical Concentration and AF is the Assessment Factor.
Concentrations that have NO Observable Effect (NOECs) for fish, aquatic invertebrates, and algal species will be used to determine the critical concentrations (CC) for water [42]. If NOEC is not present for any of these taxa, the L(E)C50 value with the lowest estimate will be used. Data from the same group of organisms reported in the Pesticides Properties DataBase of the University of Hertfordshire and the US-EPA ecotoxicological database will be used if there is no data for the aforementioned species. When toxicity information for metabolites is not available, the NOEC values of the parent pesticides will also be divided by a factor of 10 [43]. AF will be determined as follows: 10 for three species' NOECs available, 50 for two species' NOECs accessible, 100 when only one species’ NOEC data is available (fish or invertebrates), and 1000 for no NOEC dataset and an L(E)C50, will be applied [44].
For each sample site, the Total RQ will be determined using the following equation (4):
| (4) |
where RQi is the risk quotient of pesticide i.
RQ interpretation standards will be as follows: minimum risk when RQ < 0.01; low risk when 0.01 ≤ RQ < 0.1; medium risk when 0.1 ≤ RQ < 1; and high risk when RQ ≥ 1 [7,45]. The University of Hertfordshire pesticide databases will be used to obtain ecotoxicological information for all species (http://sitem.herts.ac.uk/aeru/ppdb/en/atoz.htm).
2.3.4. Health risk assessment related to eating fish from the reservoir
Only the quantified organochlorine pesticide (OCP) residues from this study will be used to evaluate the health risks linked to contaminated fish. The Food and Agriculture Organization/World Health Organisation algorithm will be employed to compute the Estimated Daily Intake (EDI) for every chemical detected in the fish samples. equation (5): EDI = (C x Fr)/Bw calculates the Estimated Daily Intake (EDI) of pesticide residue. In this equation, C represents the measured pesticide residue in grammes per kilogramme of wet weight, Fr represents the daily fish consumption in kilogrammes per day, and Bw represents the average weight of consumers in kilogrammes. In accordance with [46,47], it will be assumed that adults and children consume 0.2 kg of fish daily and have average body weights of 70 and 20 kg, respectively.
-
•
Non-carcinogenic risk
The USEPA 1991 algorithm will be used to calculate the health risk index (HRI) for each water reservoir. The purpose of this evaluation is to assess any possible health risks related to the consumption of fish contaminated with pesticides. The following equation (6) will be used:
| HRI = EDI/ADI | (6) |
Where EDI is the estimated daily intake and ADI is the Acceptable Daily Intake.
-
•
Cancer risk
The Lifetime Cancer Risk (LCR) and hazard ratios (HR) will be calculated in accordance with the standards set by the United States Environmental Protection Agency (EPA). The purpose is to assess the possible cancer-causing risks associated with pesticide residues in fish that are consumed [48]. equation (7) provided below will be utilised to compute the Lifetime Cancer Risk (LCR), which refers to the increased likelihood of developing cancer over one's lifetime as a result of continuous exposure to a cancer-causing substance [49].
| LCR = EDI x CSF | (7) |
where CSF is the cancer slope factor received from the IRIS (Integrated Risk Information System) of the USEPA (IRIS). It will be considered acceptable if the risk is < 1 in 1,000,000; between 1 in 10,000 and 1 in 1,000,000 will be deemed a cause for worry; and >1 in 10,000 will be deemed a high cancer risk [48].
-
•
Hazard ratio
The hazard ratio will be computed using the formula utilised by Jiang et al. [50] to calculate the hazard ratio for carcinogenic effects, as stated below (8):
| HR = EDI/BMC | (8) |
where BMC is the benchmark concentration for the cancer-causing effect and will be determined as follows (9):
| BMC = (Risk × Bw)/(CR × CSF) | (9) |
The risk will be set at a probability of 1 in 1,000,000 due to the lifetime of exposure and the quantity of fish consumed per unit of body weight daily (kg). Bw represents body weight, CR stands for the estimated daily fish consumption rate (in grammes per day), and CSF refers to the cancer slope factor. An HR greater than 1 indicates a possible danger to human health [51].
2.4. Statistical analysis
Statistical calculations will be conducted using the SPSS software, standard version, release 26.0 (SPSS Inc. 2008), at a significance level of 5 %. A descriptive statistic, including frequency, means, and standard deviations, will be performed to obtain a better understanding of agricultural practices and pesticide usage in agriculture in Northern Ghana. The chi-squared test of independence will be employed to quantify the relationship between variables when they are nominal. In the case of nominal and ordinal variables, the equality of proportions test will be utilised. The Sommers' test will be employed to analyse the relationship between ordinal variables. Additionally, a multiple-response analysis will be conducted for questions that allow for multiple responses. In order to assess differences in average concentrations among different fish species, water reservoirs, and between sediment and water, we will employ an analysis of variance (ANOVA) along with a two-tailed t-test. ANOVA is an appropriate statistical test for evaluating the influence of categorical independent factors on continuous outcome variables [52].
3. Discussion
This paper describes a quantitative approach with a cross-sectional design for pesticide residue monitoring and risk assessment on water, sediment and fish of two selected water reservoirs in northern Ghana. It provides information on the study methodology, the environmental and fish samples collection, the assessment of ecological risks in aquatic environments, the evaluation of health risks associated with fish consumption, and the analysis of quantitative data.
Previous studies have conducted limited study on farmers' knowledge and behaviours related to pesticide usage, providing insights into the possible consequences for aquatic ecosystems [22,53]. Pesticide contamination is a significant danger to biodiversity in agricultural environments [54]. A study conducted in Bangladesh revealed that 14 % of farmers in the Savar Upazila (SU) district and 22 % of farmers in the Mehendiganj Upazila (MU) were unaware about the potential for environmental contamination [53]. Additionally, over 17 % of farmers in SU and 34 % of farmers in MU indicated that they did not consider environmental change to be a problem. About 2 % in SU and 18 % in MU reported that they believed there was no connection between the environment and the use of agricultural pesticides. A survey conducted shown that only 7 % of farmers believed that the excessive use of herbicides could be a cause of fish extinction in water bodies [55]. The inadequate understanding and improper application of pesticides have been identified as factors that contribute to pesticide contamination. This can have detrimental effects on fish and animals in the surrounding ecosystem [56]. Similarly, Schäfer et al. [57] argued that pesticides possess the capacity to adversely impact biodiversity and its structure and functioning of ecosystems. Though these studies have offered vital insights into the beliefs and behaviours of farmers, their main focus has been on land-based habitats. As a result, there is a substantial study deficit in understanding farmers’ knowledge on water bodies contamination and pesticide use practices. Therefore, our study seeks to fill this gap by evaluating farmers' knowledge and application of pesticides in relation to aquatic ecosystems. This research is essential for comprehending the factors that contribute to pesticide contamination in aquatic environments. Through farmer interviews, we aim to clarify their understanding of the possible pollution of water bodies by pesticide remnants. This study aims to yield significant insights into the extent of contamination, the types of crops, and the primary active compounds responsible for contaminating two prominent reservoirs in northern Ghana.
The prevalence of agricultural pesticide residues in diverse environmental matrices including water bodies, sediments, and aquatic biota, has been reported in existing literature globally [7,49,[58], [59], [60], [61], [62], [63]]. These investigations have emphasised the extensive prevalence of pesticide contamination in aquatic habitats, prompting worries over its possible ecological and human health repercussions. A recent study, examined the spatial distribution, seasonal variation, and ecological risk assessment of Organophosphate pesticides (OPPs) in eutrophic estuaries in South Africa [7]. The study revealed that all 13 targeted OPPs were present in sediments from both estuaries. While 10 of them were detected in water samples. It was argued that while the water column is constantly moving, the sediment beds are usually immobile [64]. These steady conditions help in the accumulation and retention of various pesticides [7]. A study investigated the presence of 29 pesticide residues in water, sediment, and fish samples collected from Tono reservoir [59]. Among 16 organochlorines analysed, three were found in sediment samples. On the fish samples, two out of the thirteen (13) OPPs analysed were found. Additionally, three organochlorine pesticide residues were detected in the fish samples out of the sixteen analysed. This demonstrates the presence of pesticides in several components of the aquatic ecosystem. The available research has frequently concentrated on water reservoirs situated mostly in urban areas with limited agricultural operations. Therefore, there remains a need for comprehensive assessments of pesticide residues in water reservoirs located in high agricultural production rural areas. This should incorporate multiple environmental compartments within water reservoirs.
Pesticides can have harmful effects on species in aquatic environments. Research has examined the ecological hazards linked to the use of agricultural pesticides in aquatic environments, emphasising the possible dangers to aquatic biodiversity and the functioning of ecosystems [10,18,[65], [66], [67]]. These studies have recorded detrimental impacts on aquatic animals and aquatic plants, due to their exposure to pesticide residues. Therefore, previous studies have frequently concentrated on particular groups of pesticides or individual organisms, neglecting the wider ecological consequences of pesticide pollution on aquatic ecosystems. Furthermore, our comprehension of the combined effects of many pesticides on aquatic biodiversity and ecosystem health is inadequate, especially in real-world situations that involve intricate combinations of chemicals. A previous study found that chlorpyrifos, cypermethrin, and acetochlor are the pesticides that have the highest contribution to the ΣUT (Toxicity Unit) values [66]. The significant influence of chlorpyrifos on non-target organisms has the potential to alter the structure and functioning of the ecosystem [68]. Our study will generate data on the level of danger that aquatic organisms face due to pesticide utilisation and the potential health hazards for humans who consume fish from these reservoirs. Though fish and other aquafood are major providers of nutrients, they can also accumulate harmful chemicals.
Multiple systematic reviews have examined the issue of pesticide residues in food products [13,[69], [70], [71], [72]]. These reviews have shown that pesticide contamination in food, especially fish, poses a health risk. A systematic review concluded that there is a necessity for regularly conducting both retrospective and prospective cumulative risk assessments for multiple pesticide residues in food.
The study's strengths include the choice of two water reservoirs and the two most frequently consumed fish species within the community. This enhances the study's internal validity and facilitates comparison. It also utilises pesticide residue analysis methods that are both well-validated and cost-effective. The utilisation of random sampling techniques to select fish and study participants minimise the potential for selection bias. Notwithstanding the robust basis of our research, it is imperative to recognise the limitations of our study. Primarily, this stems from the adoption of a cross-sectional design, which limits the ability to demonstrate causal relationships. Nevertheless, this study contributes valuable knowledge to the field by facilitating hypothesis generation, offering descriptive insights, identifying risk factors, and establishing a foundation for future longitudinal studies. Future research should utilise designs that facilitate making inferences. Furthermore, the intended timeframe for collecting data (specifically during the rainy season) might not include the complete range of seasonal fluctuations in pesticide pollution, thereby restricting the applicability of the findings to other seasons. By specifically examining the rainy season, we aim to gain a comprehensive understanding of the difficulties and dangers related to pesticide contamination during that specific time. This will establish a fundamental basis, highlighting the significance of conducting thorough investigations throughout various seasons to understand seasonal fluctuations. In contrast, this study will offer valuable insights into agricultural practices that harm the environment and help stakeholders comprehend the risks linked to pesticide residues.
Ethical statement
This study was reviewed and approved by the Committee for Human Research, Publications, and Ethics (CHRPE), Kwame Nkrumah University of Science and Technology (KNUST) with the approval number: CHRPE/AP/864/23, dated 14/09/2023.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
CRediT authorship contribution statement
Abdou Orou-Seko: Writing – review & editing, Writing – original draft, Methodology, Investigation, Conceptualization. Dennis Chirawurah: Writing – review & editing, Methodology. Jean-Pierre Gnimatin: Writing – review & editing, Methodology. Edéya Orobiyi Rodrigue Pèlèbè: Writing – review & editing, Methodology. Joyce Aputere Ndago: Writing – review & editing, Methodology. Doris Pwatirah: Writing – review & editing, Methodology. Martin Nyaaba Adokiya: Writing – review & editing, Writing – original draft, Supervision, Methodology, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e37251.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Haggblade S., Diarra A., Jiang W., Assima A., Keita N., Traore A., Traore M. Fraudulent pesticides in West Africa: a quality assessment of glyphosate products in Mali. Int. J. Pest Manag. 2021;67:32–45. doi: 10.1080/09670874.2019.1668076. [DOI] [Google Scholar]
- 2.Imoro Z.A., Larbi J., Duwiejuah A.B. Pesticide availability and usage by farmers in the northern region of Ghana. J. Health Pollut. 2019;9 doi: 10.5696/2156-9614-9.23.190906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang W., Sandahl J., Dubois J., Flavin M., Reddy S., Neigh A., Matumba L., Gore A. Collection of data on pesticides in maize and tomato in Africa: protocol for Africa pesticide residue survey study. Bull. Environ. Contam. Toxicol. 2023;110:45. doi: 10.1007/s00128-023-03692-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajmohan K.S., Chandrasekaran R., Varjani S. A review on occurrence of pesticides in environment and current technologies for their remediation and management. Indian J. Microbiol. 2020;60:125–138. doi: 10.1007/s12088-019-00841-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.IR-4 The IR-4 Project website. 2022. https://www.ir4project.org
- 6.Kalyabina V.P., Esimbekova E.N., Kopylova K.V., Kratasyuk V.A. Pesticides: formulants, distribution pathways and effects on human health – a review. Toxicol Rep. 2021;8:1179–1192. doi: 10.1016/j.toxrep.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olisah C., Rubidge G., Human L.R.D., Adams J.B. Organophosphate pesticides in South African eutrophic estuaries: spatial distribution, seasonal variation, and ecological risk assessment. Environ. Pollut. Barking Essex 1987. 2022;306 doi: 10.1016/j.envpol.2022.119446. [DOI] [PubMed] [Google Scholar]
- 8.Olisah C., Okoh O.O., Okoh A.I. Occurrence of organochlorine pesticide residues in biological and environmental matrices in Africa: a two-decade review. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e03518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma A., Kumar V., Shahzad B., Tanveer M., Sidhu G.P.S., Handa N., Kohli S.K., Yadav P., Bali A.S., Parihar R.D. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019;1:1–16. [Google Scholar]
- 10.Yildirim I. Plant protection practises and their impact on environment. J. Cent. Eur. Green Innov. 2023;11:25–38. [Google Scholar]
- 11.Theriault V., Jiang W., Diarra A., Haggblade S., Edmund J., Ipou Ipou J., Traore A. Qualitative assessment of pesticide risks in west Africa, food secur. Policy Res. Pap. 2020:35. [Google Scholar]
- 12.Heshmati A., Komacki H.A., Nazemi F., Khaneghah A.M. Persistence and dissipation behavior of pesticide residues in parsley (Petroselinum crispum) under field conditions. Qual. Assur. Saf. Crops Foods. 2020;12:55–65. [Google Scholar]
- 13.Sadighara P., Mahmudiono T., Marufi N., Yazdanfar N., Fakhri Y., Rikabadi A.K., Khaneghah A.M. Residues of carcinogenic pesticides in food: a systematic review. Rev. Environ. Health. 2023;0 doi: 10.1515/reveh-2022-0253. [DOI] [PubMed] [Google Scholar]
- 14.Mahmoud A.F.A., Ikenaka Y., Yohannes Y.B., Darwish W.S., Eldaly E.A., Morshdy A.E.M., Nakayama S.M., Mizukawa H., Ishizuka M. Distribution and health risk assessment of organochlorine pesticides (OCPs) residue in edible cattle tissues from northeastern part of Egypt: high accumulation level of OCPs in tongue. Chemosphere. 2016;144:1365–1371. doi: 10.1016/j.chemosphere.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Opuni K.F., Asare-Nkansah S., Osei-Fosu P., Akonnor A., Bekoe S.O., Dodoo A.N. Monitoring and risk assessment of pesticide residues in selected herbal medicinal products in Ghana. Environ. Monit. Assess. 2021;193:470. doi: 10.1007/s10661-021-09261-1. [DOI] [PubMed] [Google Scholar]
- 16.Sosan M.B., Adeleye A.O., Oyekunle J.A.O., Udah O., Oloruntunbi P.M., Daramola M.O., Saka W.T. Dietary risk assessment of organochlorine pesticide residues in maize-based complementary breakfast food products in Nigeria. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e05803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ssemugabo C., Bradman A., Ssempebwa J.C., Sillé F., Guwatudde D. Pesticide residues in fresh fruit and vegetables from farm to fork in the kampala metropolitan area, Uganda. Environ. Health Insights. 2022;16 doi: 10.1177/11786302221111866. 117863022211118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yakubu F., Pelig-Ba K.B., Abagale S.A., Oseni L.A. Potential risks to the environment as a result of pesticide handling in the nanumba-north municipality, Ghana. J. Agric. Chem. Environ. 2023;12:65–83. doi: 10.4236/jacen.2023.122006. [DOI] [Google Scholar]
- 19.Abdulahi A., Obiri-Danso K., Mohammed S. Effect of farmers' attitude, usage pattern and handling of pesticides on potable water quality in northern Ghana. Int. J. Dev. Sustain. 2016;10:977–987. [Google Scholar]
- 20.Anang B.T., Fordjour E., Boateng V.F. Farmers' management practices and the quality of cocoa beans in upper Denkyira district of Ghana. Asian J. Agric. Sci. 2011;3:487–491. [Google Scholar]
- 21.Asravor R.K., Sarpong D.B. Risk preferences and management strategies of farmers in Ghana: does the type of crop grown matter? J. Int. Dev. 2023;35:1080–1098. doi: 10.1002/jid.3719. [DOI] [Google Scholar]
- 22.Abaineh A., Ejigu D., Atlabachew M., Dejen E., Tilahun G. Knowledge, attitude and practices of farmers and experts about the effects of pesticide residues on agricultural product users and ecosystems: a case of Fogera District, Ethiopia. PLoS One. 2023;18 doi: 10.1371/journal.pone.0292838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MoFA, Facts & figures: agriculture in ghana . vol. 2022. Accra; 2021. (Statistics Research and Information Directorate). [Google Scholar]
- 24.Doku B.N.A., Chen S., Alhassan E.H., Abdullateef Y., Rahman M.M. Fisheries resources of Ghana: present status and future direction. Fisheries. 2018;3 [Google Scholar]
- 25.Kuffour C., Essumang D.K., Akotoye H.K., Kuffour R.A., Abraham J.D. Pesticide and nutrient loads of lake bosomtwe in the ashanti region of Ghana. J. Water Resour. Prot. 2021;13:794–806. [Google Scholar]
- 26.Nyantakyi J.A., Wiafe S., Akoto O. Seasonal changes in pesticide residues in water and sediments from River Tano, Ghana, J. Environ. Public Health. 2022;2022 doi: 10.1155/2022/8997449. https://downloads.hindawi.com/archive/2022/8997449.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demi S.M., Sicchia S.R. Agrochemicals use practices and health challenges of smallholder farmers in Ghana. Environ. Health Insights. 2021;15 doi: 10.1177/11786302211043033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Acheampong E.O., Sayer J., Macgregor C.J., Sloan S. Factors influencing the adoption of agricultural practices in Ghana's forest-fringe communities. Land. 2021;10:266. [Google Scholar]
- 29.Cochran W.G. John Wiley & Sons; 1977. Sampling Techniques. [Google Scholar]
- 30.Nsor C.A., Obodai E.A. Environmental determinants influencing fish community structure and diversity in two distinct seasons among wetlands of northern region (Ghana) Int. J. Ecol. 2016;2016 [Google Scholar]
- 31.Kortei N.K., Heymann M.E., Essuman E.K., Kpodo F.M., Akonor P.T., Lokpo S.Y., Boadi N.O., Ayim-Akonor M., Tettey C. Health risk assessment and levels of toxic metals in fishes (Oreochromis noliticus and Clarias anguillaris) from Ankobrah and Pra basins: impact of illegal mining activities on food safety. Toxicol Rep. 2020;7:360–369. doi: 10.1016/j.toxrep.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Graaf G., Janssen H. Artificial reproduction and pond rearing of the Arican catfish Clarias gariepinus in sub-Saharan Africa. FAO Fish. Tech. Pap. 1996;No 362, Rome: FAO [Google Scholar]
- 33.OCDE Ligne directrice de l’OCDE pour les essais de produits chimiques. 2014. https://mutagenese.pasteur-lille.fr/wp-content/uploads/2020/11/gl489_140926_fr.pdf
- 34.Silva V., Alaoui A., Schlünssen V., Vested A., Graumans M., van Dael M., Trevisan M., Suciu N., Mol H., Beekmann K., Figueiredo D., Harkes P., Hofman J., Kandeler E., Abrantes N., Campos I., Martínez M.Á., Pereira J.L., Goossens D., Gandrass J., Debler F., Lwanga E.H., Jonker M., van Langevelde F., Sorensen M.T., Wells J.M., Boekhorst J., Huss A., Mandrioli D., Sgargi D., Nathanail P., Nathanail J., Tamm L., Fantke P., Mark J., Grovermann C., Frelih-Larsen A., Herb I., Chivers C.-A., Mills J., Alcon F., Contreras J., Baldi I., Pasković I., Matjaz G., Norgaard T., Aparicio V., Ritsema C.J., Geissen V., Scheepers P.T.J. Collection of human and environmental data on pesticide use in Europe and Argentina: field study protocol for the SPRINT project. PLoS One. 2021;16 doi: 10.1371/journal.pone.0259748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taft R.A., Jones C. second ed. Environmental Protection Agency; Columbus, Ohio: 2001. Sediment Sampling Guide and Methodologies. [Google Scholar]
- 36.D. of E. and S. DES Background information on the collection and preservation of sediment. 2018. https://environment.des.qld.gov.au/__data/assets/pdf_file/0030/90786/physical-and-chemical-assesssment-background-information-on-the-collection-and-preservation-of-sediment.pdf
- 37.U.S. EPA . Office of Water, U.S. Environmental Protection Agency; Washington, DC.: 2001. Methods for Collection, Storage and Manipulation of Sediments for Chemical and Toxicological Analyses: Technical Manual. [Google Scholar]
- 38.(California Department of Pesticide Regulation) CDPR Procedures for collecting water and sediment samples for pesticide analysis. 2021. https://www.cdpr.ca.gov/docs/emon/pubs/sops/fswa017.pdf
- 39.US-EPA . U.S. Environmental Protection Agency, Office of Water; Washington, D.C: 2004. Drinking Water Standards and Health Advisories. [Google Scholar]
- 40.Bundschuh M., Goedkoop W., Kreuger J. Evaluation of pesticide monitoring strategies in agricultural streams based on the toxic-unit concept—experiences from long-term measurements. Sci. Total Environ. 2014;484:84–91. doi: 10.1016/j.scitotenv.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 41.Vryzas Z., Alexoudis C., Vassiliou G., Galanis K., Papadopoulou-Mourkidou E. Determination and aquatic risk assessment of pesticide residues in riparian drainage canals in northeastern Greece. Ecotoxicol. Environ. Saf. 2011;74:174–181. doi: 10.1016/j.ecoenv.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 42.Iturburu F.G., Calderon G., Amé M.V., Menone M.L. Ecological Risk Assessment (ERA) of pesticides from freshwater ecosystems in the Pampas region of Argentina: legacy and current use chemicals contribution. Sci. Total Environ. 2019;691:476–482. doi: 10.1016/j.scitotenv.2019.07.044. [DOI] [PubMed] [Google Scholar]
- 43.Vašíčková J., Hvězdová M., Kosubová P., Hofman J. Ecological risk assessment of pesticide residues in arable soils of the Czech Republic. Chemosphere. 2019;216:479–487. doi: 10.1016/j.chemosphere.2018.10.158. [DOI] [PubMed] [Google Scholar]
- 44.Papadakis E.-N., Tsaboula A., Kotopoulou A., Kintzikoglou K., Vryzas Z., Papadopoulou-Mourkidou E. Pesticides in the surface waters of Lake Vistonis Basin, Greece: occurrence and environmental risk assessment. Sci. Total Environ. 2015;536:793–802. doi: 10.1016/j.scitotenv.2015.07.099. [DOI] [PubMed] [Google Scholar]
- 45.Hernando M.D., Mezcua M., Fernández-Alba A.R., Barceló D. Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta. 2006;69:334–342. doi: 10.1016/j.talanta.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 46.Zoumenou B.G., Aïna M.P., Imorou Toko I., Igout A., Douny C., Brose F., Schiffers B., Gouda I., Chabi Sika K., Kestemont P. Occurrence of acetamiprid residues in water reservoirs in the cotton basin of Northern Benin. Bull. Environ. Contam. Toxicol. 2019;102:7–12. doi: 10.1007/s00128-018-2476-4. [DOI] [PubMed] [Google Scholar]
- 47.Pèlèbè R.O.E., Toko I.I., Ouattara I.N., Attakpa E.Y., Jean F., Montchowui E.H., Ble C.M. Growth performance and nutritional quality of nile tilapia caged in northern Benin water reservoirs exposed to agricultural effluents. Aquac. Stud. 2020;20:191–200. [Google Scholar]
- 48.USEPA Risk assessment guidance for superfund Volume 1: human health evaluation manual (Part B), Development of risk-based preliminary remediation goals (No. EPA/540/R-92/003) 1991. https://www.epa.gov/sites/default/files/2015-09/documents/rags_a.pdf
- 49.Buah-Kwofie A., Humphries M.S. Organochlorine pesticide accumulation in fish and catchment sediments of Lake St Lucia: risks for Africa's largest estuary. Chemosphere. 2021;274 doi: 10.1016/j.chemosphere.2021.129712. [DOI] [PubMed] [Google Scholar]
- 50.Jiang Q.T., Lee T.K.M., Chen K., Wong H.L., Zheng J.S., Giesy J.P., Lo K.K.W., Yamashita N., Lam P.K.S. Human health risk assessment of organochlorines associated with fish consumption in a coastal city in China. Environ. Pollut. 2005;136:155–165. doi: 10.1016/j.envpol.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 51.Dougherty C.P., Holtz S.H., Reinert J.C., Panyacosit L., Axelrad D.A., Woodruff T.J. Dietary exposures to food contaminants across the United States. Environ. Res. 2000;84:170–185. doi: 10.1006/enrs.2000.4027. [DOI] [PubMed] [Google Scholar]
- 52.Kao L.S., Green C.E. Analysis of variance: is there a difference in means and what does it mean? J. Surg. Res. 2008;144:158–170. doi: 10.1016/j.jss.2007.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shammi M., Sultana A., Hasan N., Rahman M.M., Islam M.S., Bodrud-Doza M., Uddin M.K. Pesticide exposures towards health and environmental hazard in Bangladesh: a case study on farmers' perception, J. Saudi Soc. Agric. Sci. 2020;19:161–173. [Google Scholar]
- 54.Dormann C.F. Effects of landscape structure and land-use intensity on similarity of plant and animal communities. Glob Ecol Biogeogr. 2007;16:774–787. [Google Scholar]
- 55.Obiri B.D., Obeng E.A., Oduro K.A., Apetorgbor M.M., Peprah T., Duah-Gyamfi A., Mensah J.K. Farmers' perceptions of herbicide usage in forest landscape restoration programs in Ghana. Sci. Afr. 2021;11 doi: 10.1016/j.sciaf.2020.e00672. [DOI] [Google Scholar]
- 56.Abhilash P.C., Singh N. Pesticide use and application: an Indian scenario. J. Hazard Mater. 2009;165:1–12. doi: 10.1016/j.jhazmat.2008.10.061. [DOI] [PubMed] [Google Scholar]
- 57.Schäfer R.B., Von Der Ohe P.C., Rasmussen J., Kefford B.J., Beketov M.A., Schulz R., Liess M. Thresholds for the effects of pesticides on invertebrate communities and leaf breakdown in stream ecosystems. Environ. Sci. Technol. 2012;46:5134–5142. doi: 10.1021/es2039882. [DOI] [PubMed] [Google Scholar]
- 58.Abdel-Halim K.Y., Salama A.K., El-Khateeb E.N., Bakry N.M. Organophosphorus pollutants (OPP) in aquatic environment at Damietta Governorate, Egypt: implications for monitoring and biomarker responses. Chemosphere. 2006;63:1491–1498. doi: 10.1016/j.chemosphere.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 59.Akoto O., Azuure A.A., Adotey K.D. Pesticide residues in water, sediment and fish from Tono Reservoir and their health risk implications. SpringerPlus. 2016;5:1849. doi: 10.1186/s40064-016-3544-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barni M.F.S., Ondarza P.M., Gonzalez M., Da Cuña R., Meijide F., Grosman F., Sanzano P., Nostro F.L.L., Miglioranza K.S. Persistent organic pollutants (POPs) in fish with different feeding habits inhabiting a shallow lake ecosystem. Sci. Total Environ. 2016;550:900–909. doi: 10.1016/j.scitotenv.2016.01.176. [DOI] [PubMed] [Google Scholar]
- 61.Bhattacharjee S., Fakhruddin A.N.M., Chowdhury M.A.Z., Rahman M.A., Alam M.K. Monitoring of selected pesticides residue levels in water samples of paddy fields and removal of cypermethrin and chlorpyrifos residues from water using rice bran. Bull. Environ. Contam. Toxicol. 2012;89:348–353. doi: 10.1007/s00128-012-0686-8. [DOI] [PubMed] [Google Scholar]
- 62.Chang G.-R. Persistent organochlorine pesticides in aquatic environments and fishes in Taiwan and their risk assessment. Environ. Sci. Pollut. Res. Int. 2018;25:7699–7708. doi: 10.1007/s11356-017-1110-z. [DOI] [PubMed] [Google Scholar]
- 63.Ibigbami O.A., Aiyesanmi A.F., Adeyeye E.I., Adebayo A.O. Persistent organochlorine pesticide residues in water, sediments and fish samples from Ogbese River. Environ. Nat. Resour. Res. 2015;5:28. [Google Scholar]
- 64.Bundschuh M., Schletz M., Goedkoop W. The mode of bioturbation triggers pesticide remobilization from aquatic sediments. Ecotoxicol. Environ. Saf. 2016;130:171–176. doi: 10.1016/j.ecoenv.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 65.Danladi K.B.R., Akoto O. Ecological and human health risk assessment of pesticide residues in fish and sediments from vea irrigation reservoir. J. Environ. Prot. 2021;12:265. doi: 10.4236/jep.2021.124017. [DOI] [Google Scholar]
- 66.San Juan M.F., Lavarias S.M.L., Aparicio V., Larsen K.E., Lerner J.C., Cortelezzi A. Ecological risk assessment of pesticides in sediments of Pampean streams, Argentina. Chemosphere. 2023;313 doi: 10.1016/j.chemosphere.2022.137598. [DOI] [PubMed] [Google Scholar]
- 67.Sumon K.A., Rashid H., Peeters E.T.H.M., Bosma R.H., Van den Brink P.J. Environmental monitoring and risk assessment of organophosphate pesticides in aquatic ecosystems of north-west Bangladesh. Chemosphere. 2018;206:92–100. doi: 10.1016/j.chemosphere.2018.04.167. [DOI] [PubMed] [Google Scholar]
- 68.Huang X., Cui H., Duan W. Ecotoxicity of chlorpyrifos to aquatic organisms: a review. Ecotoxicol. Environ. Saf. 2020;200 doi: 10.1016/j.ecoenv.2020.110731. [DOI] [PubMed] [Google Scholar]
- 69.Ali S., Ullah M.I., Sajjad A., Shakeel Q., Hussain A. In: Inamuddin, Ahamed M.I., Lichtfouse E., editors. vol. 48. Springer International Publishing; Cham: 2021. Environmental and health effects of pesticide residues; pp. 311–336. (Sustain. Agric. Rev.). [DOI] [Google Scholar]
- 70.Boudebbouz A., Boudalia S., Boussadia M.I., Gueroui Y., Habila S., Bousbia A., Symeon G.K. Pesticide residues levels in raw cow's milk and health risk assessment across the globe: a systematic review. Environ. Adv. 2022;9 doi: 10.1016/j.scitotenv.2020.141830. [DOI] [PubMed] [Google Scholar]
- 71.de Andrade J.C., Galvan D., Kato L.S., Conte-Junior C.A. Consumption of fruits and vegetables contaminated with pesticide residues in Brazil: a systematic review with health risk assessment. Chemosphere. 2023;322 doi: 10.1016/j.chemosphere.2023.138244. [DOI] [PubMed] [Google Scholar]
- 72.Taiwo A.M. A review of environmental and health effects of organochlorine pesticide residues in Africa. Chemosphere. 2019;220:1126–1140. doi: 10.1016/j.chemosphere.2019.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.