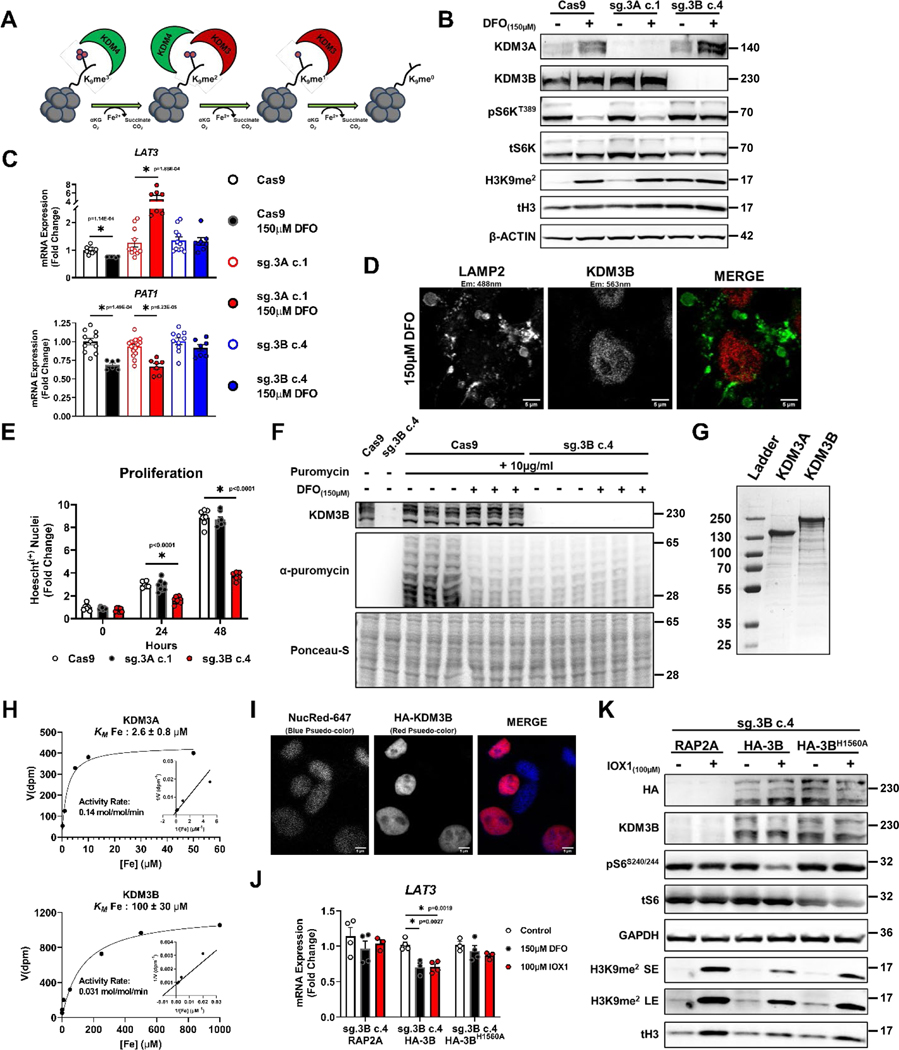
Figure 8. Regulation of mTORC1 activity by ID is mediated through KDM3B.
(A) Schematic presentation of the role of KDM3 and KDM4 family proteins in demethylating histone H3. (B) Immunoblot depicting successful editing of KDM3A and KDM3B in HEK293T cells transfected with CRISPR/Cas9 and indicated sgRNA followed by clonal selection. The experiments were performed in the presence and absence of 150μM DFO for 18 hours. Representative image of two independent experiments. (C) mRNA levels of LAT3 and PAT1 in HEK293 cell controls (Cas9), with KDM3A deletion (sg.3A c.1) and KDM3B deletion (sg.3B c.4) in the presence and absence of 150μM DFO for 18 hours. Internal control: 18S (LAT3: n=11 Cas9 control; n=6 Cas9 150μM DFO; n=11 sg.3A c.1 control; n=7 sg.3A c.1 150μM DFO; n=11 sg.3B c.4 control; n=8 sg.3B c.4 150μM DFO, PAT1: n=11 Cas9 control; n=6 Cas9 150μM DFO; n=16 sg.3A c.1 control; n=7 sg.3A c.1 150μM DFO; n=10 sg.3B c.4 control; n=7 sg.3B c.4 150μM DFO replicates, one-way ANOVA and Tukey’s post-hoc test, mean ± SE). (D) HEK293T cells stained with antibodies against LAMP2 and KDM3B in the presence of 150μM DFO for 18 hours, demonstrating the nuclear localization of KDM3B in ID. Representative image of five independent samples. (E) Quantification of viable WT, KDM3A KO, and KDM3B KO 293T cells over a 48-hour period, (n=7 replicates WT 0hr; n=8 KDM3A KO 0hr; n=8 KDM3B KO 0hr; n=6 WT 24hrs; n=8 KDM3A KO 24hrs; n=8 KDM3B KO 24hrs; n=7 replicates WT 48hrs; n=8 KDM3A KO 48hrs; n=6 KDM3B KO 48hrs;, one-way ANOVA and Tukey’s post-hoc test, mean ± SE). (F) Incorporation of puromycin into elongating peptide chains in HEK293T cells with and without KDM3B deletion and after treatment with 150μM DFO for 18 hours. Representative image of one experiment (G) SDS-PAGE gel of purified N-terminal FLAG-tagged murine KDM3A and human full-length KDM3B overexpressed using a baculoviral overexpression system. Representative image of three independent experiments (H) Enzyme kinetics of KDM3A and KDM3B in the presence of increasing concentrations of iron. (I) Nuclei staining of HEK293T cells with overexpression of HA-KDM3B to demonstrate proper nuclear localization of the protein. Representative image of five independent samples. (J) mRNA levels of LAT3 in response to 150μM DFO and 100μM IOX1 in KDM3B KO HEK293T cells with overexpression of RAP2A, WT KDM3B, and an iron-binding deficient mutant of KDM3B (KDM3BH1560A) Internal control: SNRK (n=4 replicates control RAP2A; n=4 150μM DFO RAP2A; n=3 100μM IOX1 RAP2A; n=4 replicates control HA-3B; n=3 150μM DFO HA-3B; n=4 100μM IOX1 HA-3B; n=3 replicates control HA-3BH1560A; n=4 150μM DFO HA-3BH1560A; n=3 100μM IOX1 HA-3BH1560A, one-way ANOVA and Tukey’s post-hoc test, mean ± SE). (K) Immunoblot of mTORC1 activity in KDM3B KO HEK293T cells with overexpression of WT KDM3B and KDM3BH1560A treated with 100μM IOX1 for 18 hours. Representative image of one experiment. Source numerical data and unprocessed blots are available in source data files. * indicates P value < 0.05 when noted for all panels.