Abstract
Cancer is a disease characterized by uncontrolled cell proliferation, leading to excessive growth and invasion that can spread to other parts of the body. Traditional Chinese medicine has made new advancements in the treatment of cancer, providing new perspectives and directions for cancer treatment. Rhizoma Paridis is a widely used Chinese herbal medicine with documented anti-cancer effects dating back to ancient times. Modern research has shown that Rhizoma Paridis saponins (RPS) have various pharmacological activities. RPS can inhibit cancer in multiple ways, such as suppressing tumor growth, inducing cell cycle arrest, promoting cell apoptosis, enhancing cell autophagy, inducing ferroptosis, reducing inflammation, inhibiting angiogenesis, as well as inhibiting metastasis and invasion, and these findings demonstrate the potent anti-cancer activity of RPS. Polyphyllin I, polyphyllin II, polyphyllin VI, and polyphyllin VII have been widely reported as the main active ingredients with anti-cancer properties. Polyphyllin D, polyphyllin E, and polyphyllin G have also been confirmed to possess strong anti-cancer activity in recent years. Therefore, this review dives deep into the molecular mechanisms underlying the anti-cancer effects of RPS to serve as a valuable reference for future scientific research and their potential applications in cancer treatment.
Keywords: Rhizoma Paridis saponins (RPS), Cancer, Mechanisms, Molecular targets
Graphical abstract
1. Introduction
As the global population ageing is faster, the incidence of cancer is increasing, adversely affecting the quality of life and life expectancy of patients [1]. Cancer has emerged as a leading cause of death worldwide, presenting significant challenges to society and healthcare systems, and cancer has become a significant public health issue of global concern [2,3]. In 2022, lung cancer was diagnosed as the most common cancer, resulting in nearly 2.5 million new cases, accounting for 12.4 % of global cancers followed by female breast cancer (11.6 %), colorectal cancer (9.6 %), prostate cancer (7.3 %) and gastric cancer (4.9 %). Meanwhile, lung cancer was considered as the leading cause of cancer death, with an estimated 1.8 million deaths, accounting for 18.7 %, which was followed by colorectal cancer (9.3 %), liver cancer (7.8 %), female breast cancer (6.9 %) and gastric cancer (6.8 %) [4]. In 2022, the number of new cancer cases in China was approximately 4824700 (2533900 males and 2290800 females), and the age standardized incidence rate (ASIR) of the Chinese population would be 208.58/100000 (212.67/100000 males and 208.08/100000 females). The premier five causes of cancer death (lung cancer 733300, liver cancer 316500, stomach cancer 260400, colorectal cancer 240000, and esophageal cancer 187500) was account for 67 % of the total cancer deaths [5].
Cancer is complex and involves various processes such as cell damage, inflammatory responses, cell growth, and genomic instability. The imbalance between oncogenes and tumor suppressor genes is believed to be an important factor to contribute to cancer progression. Disruption in the balance between these genes can result in abnormal cell proliferation, leading to cancer. Current cancer treatment methods include surgery, radiation therapy, chemotherapy, immunotherapy, and targeted therapy [6]. Although these treatment measures can reduce the incidence of cancer, they cannot stop the development of cancer. Additionally, the effectiveness of these treatment methods is not satisfactory, as there are risks of metastasis and recurrence after surgery, what's more, radiation therapy and chemotherapy also cause side effects in patients [7]. Developing highly effective anti-cancer methods to inhibit tumor growth, extend patient survival, and enhance quality of life is a crucial goal in cancer treatment. Therefore, it is necessary to look for new effective anti-cancer treatment methods in clinic in time. Traditional Chinese Medicine (TCM) has gained attention as an alternative therapy in cancer treatment, offering unique theoretical systems and efficacy. Chinese herbal medicines are believed to possess anti-cancer effects by intervening in cell growth, propagation, and metastasis through various pathways, as well as reducing chemotherapy and radiotherapy side effects [8,9]. It can provide cancer patients with more personalized and comprehensive treatment plans by integrating TCM and modern medicine, ultimately improving treatment outcomes, patient survival rates, and quality of life.
Rhizoma Paridis is widely used in TCM and has effects of heat-clearing and detoxifying, anti-inflammatory and pain-relieving, liver-cleansing, and calming effects [10]. With the advancement of chemical extraction methods, the active ingredients in Rhizoma Paridis are gradually being identified, including steroidal saponins [[11], [12], [13]], flavonoid glycosides [14], triterpene saponins [15], polysaccharides [16], plant sterols, flavonoids, plant ecdysone [17], and amino acids [18]. Modern pharmacological research has further demonstrated that the components or extracts of Rhizoma Paridis have favorable pharmacological activities, such as anti-cancer effects [19], antioxidant activity [20], antimicrobial effects [21], hemostatisis [22], antiparasite [23], anti-inflammation [24], immunostimulatory effects [25], antivirus [26], and uterine contraction. Particularly, Rhizoma Paridis saponins (RPS) have shown significant anti-cancer effects and played a vital role in combating cancer through various mechanisms, including inhibiting tumor cell proliferation, inducing tumor cell apoptosis, blocking tumor cell angiogenesis, and inhibiting tumor cell migration and invasion [27]. The common core configurations of RPS can be divided into four major categories, namely spirostanol, furostanol, spirostanol, and rearranged spirostanol [28]. Currently, 37 kinds of spirostanol compounds have been discovered, and mostly reported RPS in the research are spirostanol, including polyphyllin I (also named polyphyllin D), polyphyllin II, polyphyllin III, polyphyllin VI, polyphyllin VII, polyphyllin C, and polyphyllin H [29,30] (Fig. 1). Polyphyllin I, polyphyllin II, polyphyllin VI, and polyphyllin VII have been widely reported as the main active ingredients with anti-cancer properties [31]. Polyphyllin D, polyphyllin E, and polyphyllin G have also been confirmed to possess strong anti-cancer activity in recent years [[32], [33], [34]]. RPS effectively suppress a range of cancerous tumors such as lung cancer [35], gastric cancer [36], colorectal cancer [37], prostate cancer [36], and melanoma [38] by inhibiting tumor growth, accelerating cell death, alleviating inflammatory reactions, inhibiting blood vessel formation, preventing the spread and invasion of cancer cells, as well as suppressing cancer development through platelet aggregation, liver fibrosis, and immune-related signaling molecules.
Fig. 1.
Rhizoma Paridis and its anti-cancer bioactive ingredients Rhizoma Paridis saponins (RPS). Commercially available RPS include polyphyllin I/polyphyllin D, polyphyllin II, Dioscin, polyphyllin V, polyphyllin VI, polyphyllin VII, polyphyllin B, polyphyllin C, polyphyllin E, polyphyllin F, polyphyllin H, and polyphyllin G. Created with BioRender.com, FA2795Z54V.
Thus, this review summaries the latest research progress on RPS in inhibiting the activity of tumor cells and delved into how these active ingredients regulate signaling pathways and molecular targets in cancer. It helps researchers to have a more comprehensive understanding of the potential mechanisms of Rhizoma Paridis in cancer treatment and provides important theoretical basis. This review is expected to provide more scientific evidence for the clinical application of RPS for further research and development.
2. Tumor growth inhibition
The growth of tumors plays a crucial role in the formation and progression of cancer. Cancer is a disease characterized by the continuous excessive division of cells, primarily due to uncontrolled cell growth. Cancer cells have the ability to proliferate infinitely and do not exhibit the normal signs of aging which can be seen in non-cancer cells, as they grow and divide in a manner distinct from normal cells. RPS can inhibit tumor growth by inducing cell cycle arrest [39], regulating metabolism pathways [40], modulating DNA methylation [41], and regulating the Wnt/β-catenin [42] and MAPK signaling pathways [43]. MAPK pathway is a classic proliferation-related signaling pathway that can participate in a series of cellular physiological activities such as cell growth, differentiation, apoptosis, triggering tumor formation, etc. [43]. Polyphyllin VII can enhance the sensitivity of HepG2/ADR cells to doxorubicin by modulating the PI3K/Akt/MAPK signaling pathway, thereby achieving the goal of killing tumor cells [44].
2.1. Cell cycle arrest
P53 regulates cell growth, DNA repair, and cell death processes by participating in multiple cellular signaling pathways [45,46]. P21 as a downstream protein of p53 influences the cell cycle by modulating the activity of the complex between cell cycle proteins and CDKs, such as serving as the primary inhibitor of cyclin-dependent kinase 2 (CDK2) [47]. Overexpression of p21 can lead to G1 arrest, and it has been confirmed to inhibit tumor growth in vitro and in vivo [48]. Research has found that the oncogenic factor c-Jun associated with the formation and progression of tumors can induce the expression of the p21 gene. Increased generation of SP1/c-Jun complexes was observed through in-depth analysis of the p21 gene promoter, indicating that these complexes are important in the activation process of the p21 gene promoter. It has found that polyphyllin I increased the levels of c-Jun protein in a dose-dependent manner in PC9 and H1650 cells [49]. Polyphyllin I triggers the p21/CDK2/Rb pathway without affecting CDK4/6. Although polyphyllin I did not inhibit CDK4/6, it increased p21 expression and decreased the levels of CDK2 and phosphorylated CDK2. Polyphyllin I also reduced Cyclin E1 (a partner of CDK2) and downstream Rb phosphorylation without interfering with the total Rb protein [50]. Polyphyllin I has been shown to upregulate the expression of IL-6, accompanied by an increase in p21 expression [51]. The overexpression of EZH2 leads to a decrease in pro-apoptotic proteins, and polyphyllin I successfully reverses this phenomenon. When HOTAIR was reduced, the expression of EZH2 decreased, and pro-apoptotic proteins increased, thereby enhancing the therapeutic effect of polyphyllin I by inhibiting EZH2-induced cell cycle arrest and apoptosis through the STAT3/HOTAIR signaling pathway [52]. Research has shown that when HepG2 cells were treated with polyphyllin I, it exerted its effects by increasing the expression of p21 and Cyclin E1. Additionally, polyphyllin I significantly reduced the expression of Cyclin A2 and CDK2, leading to cell cycle arrest at the G2/M phase, thereby exerting inhibitory effects on tumor cells [53]. Excessive ROS induced by polyphyllin I can lead to G2/M phase arrest by modulating the expression of cell cycle regulatory factors such as p21 and Cyclin B1 [54]. Polyphyllin D can induce G2/M phase arrest in liver cells and inhibit cell growth by impairment of bile ester function. When liver cancer cells were treated with polyphyllin D, the levels of Cyclin/CDK markers (including Cyclin D3, Cyclin E1, Cyclin A2, Cyclin B1, CDK2, and CDK4) decreased, confirming the significant impact of polyphyllin D on cell cycle arrest. Polyphyllin D also sensitizes ovarian cancer cells to cisplatin-induced growth arrest [55]. The inhibitory effect of RPS on the proliferation of tumor cells may be related to their blockade of signaling pathways on the cell membrane, but the specific mechanism is not yet clear. After treatment with polyphyllin D, the amount of effective cell cycle protein-dependent kinase inhibitor p21 gradually increased, which may be one mechanism caused cell cycle arrest. In addition, polyphyllin D can induce G2/M phase arrest by inhibiting the growth of liver cancer cells [56]. Polyphyllin VII can increase the sensitivity of non-small cell lung cancer patients resistant to gefitinib by modulating the levels of p21. Studies have shown that p21 can inhibit cell cycle progression at the G1 phase by binding to CDK2/4 and cyclin proteins resulting in inhibiting their activity, thereby suppressing the growth of gefitinib-resistant cells. The combination of polyphyllin VII and gefitinib can enhance the accumulation of p21 by mediating its binding to CDK/Cyclin complexes, inhibiting the activity of CDK2/4 and Cyclin D1/E in resistant cells, and preventing the degradation of p21 [57]. Polyphyllin II significantly downregulated the protein levels of Cyclin B1, Cyclin A2, and CDC2 in colorectal cancer cells. Additionally, polyphyllin II upregulated the protein level of p21 in colorectal cancer cells, leading to cell cycle arrest at the G2/M phase [58]. Treatment of U251 glioblastoma cells with polyphyllin H induced upregulation of p21 and p27, and downregulation of the protein expression levels of Cyclin D1 and S-phase kinase-associated protein 2 (Skp2), thereby inducing cell cycle arrest at the G1 phase [59]. In conclusion, polyphyllin I, polyphyllin D, polyphyllin H and polyphyllin II can induce cancer cell cycle arrest by regulating cell cycle proteins and modulating the STAT3/HOTAIR signaling pathway (Fig. 2). Furthermore, it was observed that the cell cycle changed with a significant increase in the proportion of G1 phase and a significant decrease in the proportion of S phase in A549 cells treated with gracillin, which indicated that gracillin can inhibit cell growth leading to apoptosis [60]. Moreover, gracillin exerts inhibitory impacts on AKT phosphorylation and cell proliferation in gastric cancer BGC-823 cells. In the absence of TIPE2, the induction of TIPE2 expression and the inhibition of AKT phosphorylation by gracillin were more prominent, intimating that gracillin could inhibit gastric cancer cells through TIPE2-mediated AKT phosphorylation [61].
Fig. 2.
Illustration of Rhizoma Paridis saponins impeding tumor growth. This inhibition is achieved through the regulation of targets involved in cell cycle arrest induction, metabolic pathway modulation, DNA methylation, and the Wnt/β-catenin signaling pathway. Created with BioRender.com, UW27960QDO.
Furthermore, RPS extraction can induce G1 and G2/M phase arrest in the cell cycle [62,63]. According to research findings, RPS significantly reduced the proportion of S-phase in KYSE150 esophageal cancer cells in a dose-dependent manner, and also significantly decreased the expression of Cyclin D1. This effect leads to the inhibition of KYSE150 cell growth, and effectively prevents further development of esophageal cancer [63].
2.2. Regulation of metabolic pathways
RPS can inhibit tumor growth by modulating metabolic pathways, including glycolysis, gluconeogenesis, and amino acid synthesis. In a study, it is discovered that RPS can inhibit tumor cell growth by reversing aerobic glycolysis through the activation of tumor suppressors p53 and PTEN in H22 tumor-bearing mice with hepatocellular carcinoma (HCC). Additionally, RPS extraction reduces fat production by inhibiting fatty acid synthase (FASN), further suppressing tumor growth [41]. More importantly, RPS extraction has the ability to inhibit the expression of Myc and GLS, reduce glutamine levels, and modulate the network systems of PI3K/Akt/mTOR and HIF-1α/Myc/Ras, which are involved in metabolic processes, effectively preventing tumor growth [41]. Another scientific study has shown that RPS extraction has the ability to inhibit the conversion of glucose and valine into ketones, thereby participating in the process of ATP generation. This action limits the oxidative pathways of fatty acids and sugars, interrupting the energy supply to tumor cells. RPS extraction can also inhibit the generation of glycine and alanine, further preventing the progression of tumors [64]. The study found that extracelluar acidification rate (ECAR) and lactic acid production were significantly reduced after triple negative breast cancer cells treated with gracillin. Furthermore, the research has shown that gracillin can inhibit the activity of phosphoglycerate kinase 1 in the glycolytic pathway [65].
2.3. Regulation of DNA methylation
In addition to changes in tumor metabolism, DNA methylation plays a significant role in the development and progression of cancer and can be considered as a biomarker for cancer diagnosis and early screening [66]. DNA methyltransferases (DNMTs) are an important class of enzymes, whose core function is to transfer methyl groups to cytosine residues of CpG, directing the entire process of DNA methylation [67]. DNMT1, DNMT3A, and DNMT3B are excessive expression in cancer patients’ tumor tissues, where their expression levels significantly higher than those in non-tumor tissues [68]. Transcription of DNMT genes can be induced by the Ras-c-Jun signaling pathway, Sp1 and Sp3 zinc finger proteins, Wilms' tumor 1, homeobox B3, and other human viruses [40]. Research shows that polyphyllin I can enhance the phosphorylation of stress-activated protein kinase (SAPK)/c-Jun N-terminal kinase (JNK) triggered by stress when reducing the expression levels of p65 and DNMT1 proteins. When JNK phosphorylation is triggered, it has the ability to have the effect on downstream transcription factors (JUN, ELK1, ET) and promote activities such as cell growth, differentiation, survival, and apoptosis [69]. Due to the decreased expression of p65 and DNMT1 proteins, the expression of the enhancer of zeste homolog 2 (EZH2) decreased. DNMT1 and EZH2 as highly active genes in cancers is expected to become new targets for future cancer therapy [49,69,70]. Temozolomide (TMZ) is a commonly used chemotherapy drugs for glioma patients. Unfortunately, this substance is somewhat limited due to the severe side effects and the drug resistance caused by the expression of O6-methylguanine-DNA methyltransferase (MGMT). The combination of TMZ and polyphyllin generates significant synergistic effects during the treatment process [71]. MGMT is an enzyme that participates in transferring a methyl group from the O6 position to a cysteine residue, effectively repairing mutations in tumor cells before DNA replication. High levels of MGMT expression can lead to tumor cell resistance to TMZ, thus reducing the efficacy of TMZ [72]. Polyphyllin VII can reduce TMZ resistance by inhibiting MGMT expression [73].
2.4. Regulation of the Wnt/β-catenin signaling pathway
The Wnt/β-catenin signaling pathway is involved in the proliferation of tumor cells. Numerous scientific studies have confirmed that the Wnt/β-catenin signaling pathway is one of the key oncogenic pathways leading to the occurrence and progression of osteosarcoma [74]. β-catenin is the intracellular signaling mediator of the Wnt/β-catenin signaling pathway, and it can regulate cadherin-catenin complex [75]. In addition, it is vital in the activation of the Wnt/β-catenin signaling pathway during embryo development and tumor formation processes [76]. When GSK-3β is phosphorylated at Ser9, β-catenin will not be phosphorylated and lose its activity [[76], [77], [78]]. Polyphyllin I can deactivate the Wnt/β-catenin pathway both in vitro and in vivo, effectively inhibiting the growth of human osteosarcoma. The levels of p-GSK-3β decreased after treatment with polyphyllin I, further inhibiting the accumulation of active β-catenin. This dynamic change leads to a unique regulatory state of β-catenin, when β-catenin is phosphorylated, it can be ubiquitinated and further degraded by proteasomes [42].
Polyphyllin VII is an innovative moesin inhibitor with the ability to inhibit the proliferation of multiple myeloma cells and overcome resistance to bortezomib. Moesin, the target of polyphyllin VII, is a core regulatory factor in the Wnt/β-catenin pathway. Polyphyllin VII binds to moesin and targets it for degradation through the ubiquitin-proteasome pathway. This process disrupts the activity of the Wnt/β-catenin pathway, leading to a decrease in myeloma cell proliferation and solving the problem of bortezomib resistance [79].
Tumor growth is mainly due to cancer cells proliferating unlimitedly in the human body. Normal tissues exhibit precise regulation over the generation and release of growth-promoting signals, which can guide the initiation and progression of cell proliferation and differentiation cycles, maintaining a balance in cell numbers and ensuring the structure and function. Cancer cells typically lose sensitivity to these regulatory signals or autonomously produce excessive signals promoting cell proliferation, leading to their unlimited proliferation and tumor formation. RPS can achieve the goal of inhibiting tumor growth by inducing cell cycle arrest, regulating metabolic pathways, regulating DNA methylation, and regulating the Wnt/β-catenin signaling pathway.
3. Promotion of cell death
Regulated cell death (RCD) is a cell death mode that can be regulated by various biological macromolecules and differs from accidental cell death (ACD) [80]. RCD that occurs in a normal physiological environment is also referred to as programmed cell death (PCD). RCD mainly includes autophagy-dependent cell death, apoptosis, necroptosis, pyroptosis, ferroptosis, parthanatos, entosis, NETosis, lysosome-dependent cell death (LCD), alkaline lipid accumulation disease, and oxeiptosis [81]. During the process of RCD, different lethal subprograms can have an impact on the development and treatment response of cancer. The resistance of cancer cells may lead to treatment failures [82,83]. In the early stages of cancer, cancer cells may exhibit resistance to cancer treatment by disrupting the RCD pathway caused by mutations [84]. The prevention of RCD is considered as an important indicator of cancer, and RPS can inhibit cancer by promoting cell death (Fig. 3).
Fig. 3.
Visualization of Rhizoma Paridis saponins promoting cancer death. The process of cell death is intricate, with these saponins hindering tumor progression by targeting molecules that facilitate apoptosis, autophagy, and ferroptosis in cancer cells. Created with BioRender.com, RP279613L1.
3.1. Apoptosis induction
Mitochondria play a central role as core regulatory organelles in the process of cell apoptosis, and the release of cytochrome c is an important step in apoptosis. The released cytochrome c forms a complex by binding with apoptotic protease-activating factor 1 (Apaf-1) in the presence of dATP. This leads to the activation of caspase-9, which in turn activates caspase-3 and other caspases, triggering cell apoptosis [85]. Cysteine aspartate protease, especially caspase-3, is one of the key mediators during the process of apoptosis. Caspase-3 is a death protease that is frequently triggered and can specifically cleave essential cellular proteins. The Bcl-2 family includes anti-apoptotic members (such as Bcl-2 and Bcl-xL) and pro-apoptotic members (such as Bax and Bak), playing vital regulatory and modulating roles in the process of apoptosis. It is worth emphasizing that a high Bcl-2/Bax ratio is considered as a key factor in resisting the apoptosis process. The dose-dependent increase of polyphyllin I enhanced the release of mitochondrial cytochrome c, while also elevating levels of Fas, p53, p21, and the Bax/Bcl-2 ratio [53]. Additionally, polyphyllin I reduced the inhibitory effect of lncRNA-ROR on tumor growth, and induced intrinsic and extrinsic apoptosis in nasopharyngeal carcinoma cells, thereby enhancing p53 signaling transduction [86]. In addition to the above functions, the cleavage of caspase-3, -8, and -9 were also be triggered, followed by the subsequent cleavage process of poly (ADP-ribose) polymerase (PARP). These events collectively participate in regulating the process of apoptosis in cells [86]. Patients with decreased cell proliferation and increased cell apoptosis levels are often associated with an increase in the pro-apoptotic protein Bax. Studies have found that the expression of Bcl-2 protein decreased while Bax increased, meanwhile, the expression levels of caspase-3 protein and the Bax/Bcl-2 ratio significantly increased after polyphyllin I treatment [87]. The research findings confirm the therapeutic effect of polyphyllin I on HCC. The study revealed that polyphyllin I can inhibit the PI3K/Akt signaling pathway and effectively suppress the growth and spread of HCC by activating both cysteine-dependent and -independent apoptotic pathways [88]. The apoptosis-promoting elements consisting of Bax, cytochrome c in the cytoplasm, activated caspase-3, and activated caspase-9 showed an increasing trend in human ovarian cancer cells (SKOV3) after the treatment of polyphyllin II. Furthermore, polyphyllin II treatment also reduced the phosphorylation level of the extracellular signal-regulated kinase (ERK1/2) and the expression of anti-apoptotic protein Bcl-2, promoting the apoptosis process [89]. Research has shown that the activity of the anti-apoptotic protein Bcl-2 decreased with the increase of polyphyllin II concentration, while the activity of the pro-apoptotic protein Bax increased, resulting in an increase in the ratio of Bax/Bcl-2. This indicates that the complexity of regulating the cell apoptosis pathway involved multiple complex molecular interactions. It is also observed that the protein expression level of cleaved caspase-3 slightly decreased after treatment with polyphyllin II [90]. Gracillin inhibits the growth of human leukemia HL60 cells and causes cell cycle arrest in the G1 phase. Cells were treated with gracillin resulted in a notable increase in malondialdehyde (MDA) and a dose-dependent reduction in superoxide dismutase (SOD). Concurrently, the expression level of Bcl-2 decreased while the expression level of Bax increased, indicating that gracillin may induce apoptosis by enhancing intracellular oxidative stress and G1 cell cycle arrest [91]. Additionally, gracillin impaired mitochondrial mediated cellular bioenergy by inhibiting the mitochondrial function mediated by Complex II (CII) resulting in a decrease in mitochondrial membrane potential, oxidative phosphorylation, and adenosine triphosphate (ATP) synthesis, and an increase in the generation of reactive oxygen species (ROS) in cancer cells, consequently promoting apoptosis. This implies that gracillin could potentially become a targeted anti-tumor drug that has an impact on mitochondrial function [92]. One scientific study showed that polyphyllin D induced cell apoptosis through the mitochondrial apoptosis pathway. Specifically, it is characterized by decreased expression levels of Bcl-2 and Bcr/Abl, disruption of mitochondrial membrane potential, and increased levels of Bax, cytochrome c, and cleaved caspase-3. Additionally, polyphyllin D at lower doses can enhance the expression of CD14 on the surface of K562 cells and promote cell differentiation towards monocytes or mature macrophages [93]. The study found that polyphyllin G significantly increased the cleavage of caspase-3, caspase-8, caspase-9, and PARP in nasopharyngeal carcinoma cells in a dose-dependent manner in 24 h. Besides, polyphyllin G significantly reduced the expression levels of Bcl-2 and Bcl-xL proteins, increased the expression level of Bax proteins, leading to apoptosis of cancer cells [94]. The activity of proteins related to cell apoptosis (such as Fas, caspase-3, -8, -9, and PARP) was studied after treating HepaRG cells extracted from human liver cancer cells with polyphyllin VI. Compared to the control group, a slight increase in Fas expression level was observed after 24 h when treated with polyphyllin VI. Also, the expression of PARP (as a substrate of caspase-3) was increased, which led to biochemical and morphological changes in the cells. The expression levels of caspase-3, -8, and -9 increased, indicating the activation of caspase enzymes and initiation of cell apoptosis signals. These research findings suggest that polyphyllin VI induces apoptosis in HepaRG cells through both extrinsic and intrinsic apoptotic pathways [95]. According to research findings, polyphyllin VI can induce a transition from apoptosis to pyroptosis by activating caspase-1 in A549 and H1299 cells, ultimately leading to cell death. This process is closely associated with the ROS (reactive oxygen species)/NF-κB (nuclear factor-kappa B)/NLRP3 (NOD-like receptor protein 3)/GSDMD (gasdermin D) signaling axis [96]. Natural products such as polyphyllin I, polyphyllin II, polyphyllin D, polyphyllin G, and polyphyllin VI can induce apoptosis by regulating the levels of Bax/Bcl-2 ratio and the expression of caspases in cells.
A scientific study has found that polyphyllin I can inhibit the viability of gefitinib-resistant non-small cell lung cancer (NSCLC) cells and xenograft models, further inducing cell apoptosis. This therapeutic effect is closely associated with the downregulation of metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) during metastasis of human lung adenocarcinoma, leading to the inactivation of the STAT3 signaling pathway. The JAK2/STAT3 signaling transduction pathway involves multiple processes such as physiological and pathological responses, cell growth, differentiation, and death [97]. When JAK2 is activated, it triggers the phosphorylation of STAT3. The inhibitory and apoptotic effects of polyphyllin I on gefitinib-resistant NSCLC cells are counteracted by the overexpression of MALAT1, while the inhibitory and apoptotic effects of polyphyllin I are enhanced by the inhibition of MALAT1. These findings suggest that polyphyllin I effectively inhibits the activity of gefitinib-resistant NSCLC cells by reducing MALAT1 and inhibiting the STAT3 signaling pathway, thereby inducing the process of cell apoptosis both in vitro and in vivo [98]. Another scientific study has shown that p-STAT3 can bind to the promoter of its target genes, activating the transcription and expression of related genes. The study demonstrated that polyphyllin I can effectively inhibit the phosphorylation of STAT3 in a concentration-dependent manner in a mouse model with gastric cancer [99]. Excessive generation of ROS may lead to induction of apoptosis resulting from an increase in apoptosis-related proteins. The role of polyphyllin VI in inducing ROS generation in glioma cells was investigated using the fluorescent probe DCFH-DA, and the research data showed that polyphyllin VI can induce ROS production and activate JNK and p38 when treating glioma cells [100].
RIP3 (also known as RIPK3) is a serine/threonine protein kinase that can trigger two parallel cell death pathways, necroptosis and apoptosis [101]. Necroptosis is a form of cellular self-defense mechanism that is triggered when it is inhibited. This process is mediated by the RIPK1-RIPK3-MLKL signaling cascade, which is the downstream of tumor necrosis factor-alpha (TNF-α), Fas or TRAIL ligands, and Toll-like receptors [102]. Activation of RIPK3 kinase is required to promote the phosphorylation of MLKL at Ser358 in human, whereas the phosphorylation site of MLKL is at Ser345 in mice [103]. The TAM (Tyro3, Axl, and Mer) receptor tyrosine kinase family members have been widely acclaimed for their outstanding anti-apoptotic, pro-cancer, and anti-inflammatory properties. In many cell model studies, TAM kinases are able to effectively inhibit necroptosis and cell death. Phosphorylation of MLKL at Tyr376 by TAM kinases has been identified as a critical node in the involvement of TAM kinases in necroptotic signaling [104]. In one study, two different human neuroblastoma cell lines were used to establish subcutaneous tumor models in mice. Polyphyllin D as a treatment option utilized immunohistochemistry to observe its effects on normal tissues and metastatic tumor cells to explore the mechanism. The research indicated that polyphyllin D significantly increased the sensitivity of cancer cells to chemotherapy. Polyphyllin D induced the expression of RIPK3 with different responses observed at Ser358 in IMR-32 cell line as well as at Ser358 and Tyr376 in LA-N-2 cell line in human neuroblastoma. It suggests that polyphyllin D has a notable inhibitory effect and leads to necrotic apoptosis in cancer cells [105]. CIP2A is a protein phosphatase that is also considered as an oncogene, regulating several key oncogenic signaling proteins including Akt, c-Myc, ERK, and p70S6K. CIP2A promotes cell transformation and tumor formation by inhibiting the activity of PP2A. Research has shown that polyphyllin I reduced the activity of the CIP2A inhibitor, leading to the degradation of CIP2A protein, decreased expression levels of p-Akt, and the death of gastric cancer cells [36]. Polyphyllin I exerted inhibitory effects on CIP2A/PP2A signaling, leading to reduced expression levels of p-ERK, thereby decreasing epithelial-mesenchymal transition (EMT) of prostate cancer cells and inducing apoptosis [106].
In addition, RPS extraction can reduce the levels of inflammatory cytokines, TNF-α, IL-10, and IL-8 in the serum of C57BL/6 mice via immune modulation, leading to nuclear changes such as DNA condensation and chromatin fragmentation in A549 cells, resulting in cell apoptosis [13] (Fig. 3).
3.2. Autophagy enhancement
Autophagy has been confirmed as a newly discovered programmed cell death phenomenon, which is the process of cellular self-digesting, playing a vital role in maintaining tissue homeostasis [107]. The PDK1-Akt-mTOR signaling pathway can regulate autophagy and apoptosis processes in tumor cells [108]. AMP-activated protein kinase (AMPK) is considered as the most important sensor of cancer cell energy status, which can prevent rapid proliferation and metabolism of tumors. When AMPK is activated, TSC2 and RAPTOR are phosphorylated, leading to the downregulation of the mammalian target of rapamycin complex 1 (mTORC1). Activation of AMPK phosphorylates ULK1, increasing its activity and promoting the autophagy process [109]. When autophagy is initiated, a small peptide of cytosolic LC3-I is cleaved off, which conjugates with phosphatidylethanolamine (PE) to form the membrane-bound LC3-II. Therefore, estimating the ratio of LC3-II/I can evaluate the level of autophagy. LC3-II is considered as a marker of autophagy and an important regulatory factor in the process. In mammals, there are four isoforms of LC3 (LC3A, LC3B, LC3B2, and LC3C), each with I and II subtypes. After synthesis, LC3 protein is cleaved by Atg4 to expose a glycine residue, forming the cytosolic LC3-I. During autophagy, LC3-I undergoes ubiquitin-like conjugation and processing to be coupled with PE to form LC3-II, which then localizes on the inner and outer membranes of autophagosomes. As autophagosomes form, cytosolic LC3-I binds to PE and is transferred to the double-membrane of autophagosomes, forming the membrane-bound LC3-II [110]. The conversion from LC3-I to LC3-II is a crucial step in the autophagy process. P62 is another known protein that plays an important role in autophagy. Results showed that polyphyllin I led to a dose-dependent increase in the expression of LC3-II in HGC-27 cells, while the expression of p62 showed a decreasing trend. It indicates that polyphyllin I is involved in the autophagy process [111]. The subsequent results confirmed that polyphyllin I reduced the phosphorylation levels of mTORC1 effectors (mTOR, p70S6K, and 4E-BP1) in an AMPK-dependent manner, significantly inhibiting the Akt/mTOR signaling pathway and downstream effectors. There are several studies reported that polyphyllin I induced autophagy through the AMPK/mTOR signaling transduction pathway [31,112,113]. Polyphyllin I can inhibit the Akt/mTOR pathway through ROS and promote autophagy, cell death, and apoptosis in colon cancer cells [114]. It is demonstrated that polyphyllin G significantly increased the autophagy biomarkers expression (LC3I/II, Beclin-1and p62) and reduced the expression of PI3K, activated mTOR (ser2448), total mTOR, Raptor, Rictor, and GβL in a dose-dependent manner [94]. Research has shown that polyphyllin II can lead to a decrease in the levels of p-Akt and p-mTOR proteins in colorectal cancer cells. Further studies indicate that the autophagy induced by polyphyllin II is associated with the inhibition of the PI3K/Akt/mTOR signaling pathway. In the presence of the mTOR inhibitor rapamycin, the effects of polyphyllin II on p-mTOR decrease and LC3B-II increase in colorectal cancer cells further confirmed polyphyllin II realizing autophagy via Akt/mTOR pathway [58]. Gracillin can significantly upregulate the expression levels of FAM102A and Beclin-1, downregulate the expression level of p62, inhibit the phosphorylation of PI3K and Akt to enhance autophagy process [60]. It is also showed that gracillin can regulate autophagy behavior via mTOR signaling pathway [115]. Research has shown that when glioma cells were exposed to polyphyllin VI, the expression of LC3-II increased. When glioma cells treated with polyphyllin VI, Beclin-1 (another autophagy-related protein) was also upregulated, suggesting that polyphyllin VI was involved in inducing autophagy in glioma cells [100]. Polyphyllin VI can promote the generation of ROS and activate the JNK and P38 pathways. Previous studies have indicated that excessive production of ROS may trigger the autophagy process. This suggests that polyphyllin VI may promote the process of cellular autophagy through the ROS-mediated mechanism [116]. The formation of acidic vesicular organelles (AVOs) is a characteristic of autophagy [117], and polyphyllin VII increased the expression of AVOs in a dose-dependent manner. It is widely believed that LC3 promotes the formation of autophagosomes and activates the Atg5-Atg12 complex, thereby facilitating the binding of LC3I. These results suggest that polyphyllin VII can promote the process of autophagy by affecting the expression of autophagy genes and the formation of autophagosomes [118]. Polyphyllin VII upregulated LC3II, Atg7, Atg5, and Atg12-Atg5 complex to induce autophagy [118], while polyphyllin G induced the formation of AVO in nasopharyngeal carcinoma cells [94].
Chaperone-mediated autophagy (CMA) is a selective autophagy mechanism specific to protein degradation that effectively disrupts the degradation process by inhibiting the interaction between HSC70 and LAMP2A [119]. Polyphyllin D has been identified as a small molecule inhibitor of CMA by disrupting the interaction of HSC70-LAMP2A. Polyphyllin D binded to the nucleotide-binding domain of HSC70 as well as the E129 and T278 residues at the C-terminus of LAMP2A. Polyphyllin D induced the accumulation of ROS by inhibiting the HSC70-LAMP2A-eIF2α signaling axis to accelerate the production of unfolded proteins. Furthermore, polyphyllin D cut off the STX17-SNAP29-VAMP8 signaling axis to prevent cellular compensatory mechanisms induced by CMA inhibition [120].
Based on the above research, we have learned that RPS can enhance cell autophagy capacity through various mechanisms. It mainly includes enhancing the PDK1-Akt-mTOR signaling pathway, increasing the activity of autophagic protein p62, inhibiting the expression of LC3- II, suppressing PI3K/Akt/mTOR signaling transduction, inducing the formation of AVOs, and interrupting the signal transduction axis of STX17-SNAP29-VAMP8 (Fig. 3).
3.3. Ferroptosis induction
Ferroptosis is a newly emerging iron-dependent programmed cell death characterized by lipid peroxidation and iron accumulation. Reduced glutathione (GSH) is important in this process by converting harmful lipid peroxides into non-toxic lipid alcohols, preventing the occurrence of ferroptosis [121]. More and more scientific studies indicate that GPX4 belonging to the selenocysteine enzyme family is of significance in many common malignant tumors [122]. Polyphyllin B, a novel small molecule, has been identified as GPX4 inhibitor. In the research model, polyphyllin B can directly bind to GPX4 and reduce its expression. Besides, it regulated iron ion metabolism by promoting iron ion transport and ferritin engulfment to increase Fe2+ activity, and ultimately leading to ferroptosis. Mitochondrial shrinkage, reduced mitochondrial ridges, and autophagic vacuoles were observed in gastric cancer cells treated with polyphyllin B. These observations suggest that polyphyllin B has the ability to induce ferroptosis in gastric cancer cells in vitro [123].
Nuclear factor erythroid 2-related factor 2 (NRF2), as a specific transcription factor, involves in reducing lipid peroxidation and iron-mediated cell death processes [124]. Ferritin heavy chain 1 (FTH1) is not only a major subunit of ferritin, but also a key regulator of iron-mediated cell death [125]. According to research, the intervention of polyphyllin I can induce ferroptosis by enhancing mitochondrial damage and activating the Nrf2/HO-1/GPX4 axis. Findings showed that HCC cells treated with polyphyllin I led to elevated levels of ROS and malondialdehyde (MDA), resulting in dose-dependent inhibition of proliferation, invasion, and migration. This inhibitory effect promoted the accumulation of Fe2+ in the cells, depleted reduced GSH, and suppressed the expression of xCT and GPX4, ultimately triggering iron ferroptosis. The induction of ferroptosis by polyphyllin I is closely associated with its interaction with Nrf2, HO-1, and GPX4 proteins, further affecting the function of the Nrf2/HO-1/GPX4 antioxidant axis. In in vivo studies, polyphyllin I was capable of inhibiting ferroptosis caused by the Nrf2/HO-1/GPX4 axis, suppressing cell growth, and exhibiting similar effects to sorafenib [126]. According to research, polyphyllin I effectively reduced the expression levels of factors related to NRF2 and FTH1 in AGS and MKN-45 gastric cancer cells. Further studies have shown that the ferroptosis inhibitor Liproxstain-1 largely neutralizes the regulatory effect of polyphyllin I on these factors. This indicates a close relationship between ferroptosis induced by polyphyllin I and the NRF2/FTH1 pathway. Specifically, polyphyllin I affects the ferroptosis process by modulating the expression levels of NRF2 and FTH1 [127]. Furthermore, research has found that polyphyllin I increased the expression level of miR-124-3p in tumor tissues both in vitro and in vivo. MiR-124-3p regulated ferroptosis by directly targeting the 3′-UTR region of NRF2, leading to a decrease in NRF2 level and ultimately resulting in ferroptosis [128]. The above results indicate that polyphyllin I mainly exerts its anti-cancer effect via the ferroptosis Nrf2-related signaling axis.
Extensive studies have shown that there is a close interaction between autophagy and ferroptosis. Excessive autophagy in cells can lead to iron accumulation, thereby accelerating the process of iron-dependent cell death. Ferritinophagy is a selective autophagic degradation process targeting ferritin proteins, primarily aimed at releasing free iron to promote intracellular iron accumulation. The iron-specific autophagic process can induce oxidative damage through the Fenton reaction, triggering iron-dependent cell death [129]. The nuclear receptor coactivator 4 (NCOA4) acts as a transmembrane receptor in the process of ferritin degradation, the C-terminal domain of which binds to ferritin, facilitating the transfer of ferritin to autolysosomes for degradation. This process promotes the release and recycling of iron, maintaining intracellular iron homeostasis and regulating iron metabolism processes [130]. In the case of ferroptosis, the autophagic mechanism of mitochondria becomes complicated. In the early stages of iron overload, a large amount of free iron enters the mitochondria, and the autophagy of the mitochondria isolates the iron in the autophagosome, thereby reducing the generation of ROS. However, as the iron overload worsens, the mitochondria are damaged, their autophagic ability is further enhanced, and the previously isolated iron begins to be released massively, leading to an excessive generation of ROS and lipid peroxidation. It further triggers multiple cell death pathways, including ferroptosis caused by ROS-induced cell death pathways. The intricate relationship between mitochondrial autophagy and ferroptosis reveals the crucial regulatory mechanism of cellular iron metabolism balance for cell survival [131]. Autophagy and ferroptosis are connected at the molecular level, with distinct types of autophagy such as ferritinophagy, mitophagy, lipophagy, and clockophagy contributing to ferroptosis [132]. T-LAK cell-originated protein kinase (TOPK) is a serine-threonine, the overexpression of which promotes tumor development and progression in gastric cancer [133]. RPS exerting anti-tumor effects by effectively inhibiting the TOPK signaling pathway. Polyphyllin VII induced autophagy-dependent ferroptosis by targeting TOPK, which triggers iron-dependent cell death. The autophagic upstream of Unc-51-like autophagy activating kinase 1 (ULK1) was activated by polyphyllin VII, which inhibited the activity of TOPK resulting in reducing its constraining effect on downstream ULK1 realized by directly binding to the stable non-active dimer form of TOPK [134]. Polyphyllin VII inhibited the generation of gastric cancer by triggering autophagy-mediated ferroptosis and by contributing to the degradation of FTH1 primarily responsible for ferroptosis during autophagy, eventually enhancing autophagic iron formation and reducing its toxicity.
It is learned that cell death is a complex process based on in-depth research recent years. Many studies have explored the role of cell death in cancer tumors and provided new insights for therapeutic approaches. In sum, studies mentioned above have shown that RPS can inhibit tumor development by inducing apoptosis, autophagy, and ferroptosis in cancer cells (Fig. 3).
4. Reduction in inflammation
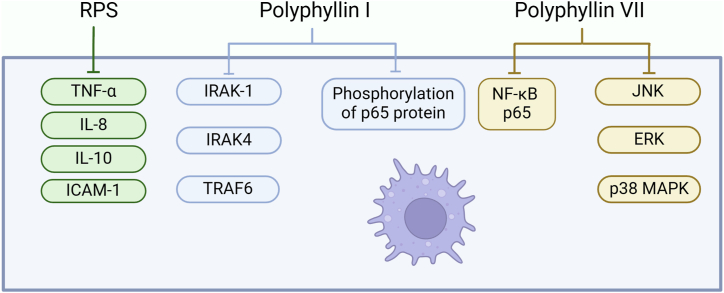
In the process of inflammation, NF-κB signaling transduction is considered to be one of the main transcription pathways. In macrophages, NF-κB is activated by Toll-like receptors (TLRs), leading to the reverse activation of cell factors, chemokines, and other pro-inflammatory mediators. Activated macrophages of importance in host pathogen infection by producing inflammatory factors and pro-inflammatory mediators, such as nitric oxide (NO), prostaglandin E2 (PGE2), inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), metalloproteinases, TNF-α, interleukin (IL)-1β, and IL-6, which recruit more immune cells to the site of infection or tissue damage [[135], [136], [137]]. In the traditional signaling pathway, strong stimuli such as lipopolysaccharide (LPS), interferon-gamma (IFN-γ), and TNF-α can activate the NF-κB (IκB) kinase complex (IKK), leading to phosphorylation and degradation of IκBα protein at serine residues. This process releases NF-κB, which then enters the nucleus to regulate the transcription of target genes including TNF-α, IL-1β, IL-6, and iNOS [[138], [139], [140]]. Polyphyllin I inhibits macrophage inflammatory response by blocking the NF-κB pathway. In the innate immune system, macrophages respond to external stimuli such as LPS by activating surface TLRs. In the initiation and perpetuation of inflammation in rheumatoid arthritis (RA), TLR activation triggers signaling pathways including dimerization. Besides the TLR3 signaling pathway, other TLR signaling pathways also recruit adaptor molecule MyD88, which then recruits downstream signaling mediators (such as IRAK-1, IRAK4, and TRAF6) to form a receptor complex. Polyphyllin I has a certain impact on the anti-NF-κB pathway downstream of TAK1 [141]. NF-κB p65 is an important subunit of NF-κB, of great importance in regulating biological processes such as inflammation, immune response, and cell apoptosis. It is reported that polyphyllin I can inhibit the platinum-induced phosphorylation of NF-κB p65 protein and the expression of its downstream genes, thereby increasing the sensitivity of liver cancer to chemotherapy drugs [142]. Pre-treatment with polyphyllin VII successfully reduced the cytoplasmic translocation and nuclear translocation of NF-κB p65 protein under lipopolysaccharide stimulation. Additionally, lipopolysaccharide treatment significantly enhanced the phosphorylation level of IκB-α, while the total amount of IκB-α in macrophages was reduced. This indicates that IκB-α is phosphorylated and degraded via the ubiquitin-proteasome system, but its phosphorylation process is markedly inhibited. The degradation process is partially reversed by treatment with polyphyllin VII by inhibiting the activity of JNK, ERK, and p38 MAPK induced by lipopolysaccharide [143]. Clinical studies have shown that inflammation-related factors are involved in the risk of cancer, and cytokines form a pro-inflammatory cytokine network through secretion, functional regulation, and interactions in various cell population [144]. The levels of IL-8 and IL-10 in the serum of NSCLC patients were significantly higher than those in normal individuals [145],which can be reversed by RPS treatment. It is explained that RPS treatment can reduce the expression of pro-inflammatory cell factors MCP-1, IL-6, TGF-β1, and cell adhesion molecule ICAM-1 [13](Fig. 4).
Fig. 4.
Visualization of Rhizoma Paridis saponins inhibiting inflammation. Rhizoma Paridis saponins inhibit tumors by reducing the activity of inflammatory factors and inhibiting the NF-κB signaling pathway. Created with BioRender.com, UY27961FZL.
In conclusion, inflammation increases the risk of cancer development, and tumor cells regulate the inflammatory process by secreting inflammatory mediators in the tumor microenvironment. Inflammation controls the occurrence, prognosis, and treatment process of cancer. RPS have the ability to inhibit tumors by reducing the activity of inflammatory factors and inhibiting the NF-κB signaling pathway.
5. Inhibition of invasion and metastasis
The spread of tumor cells is a dangerous process in the development of tumors. While normal cells undergo orderly proliferation and differentiation, tumor cells exhibit abnormal proliferation, losing the balance of normal growth regulation and leading to rapidly uncontrolled proliferation and metastasis. This abnormal proliferation triggers a series of disruptions, such as angiogenesis, extracellular matrix (ECM) degradation, and dysregulation of anti-apoptotic mechanisms, which contribute to the invasiveness and metastatic potential of tumors [146]. When tumor cells form a clonal structure in distant organs, it often causes significant damage to the human body. Cancer cells undergo EMT, resulting in changes in morphology and acquiring enhanced motility and invasive capabilities. The invasiveness of tumors during their development is mainly determined by the complex biochemical and biological changes in the tumor cells themselves and their associated stroma [147]. During the process of EMT, cancer cells transit from tightly packed epithelial cells to active and motile mesenchymal cells. Cancer cells must abandon many characteristics of epithelial cells, change their morphology, detach from the epithelial layer, and undergo a series of notable transformations in order to gain better motility and invasive capabilities [148]. EMT is essential in the entire process of cell invasion and metastasis (Fig. 5).
Fig. 5.
Depiction of Rhizoma Paridis saponins inhibiting tumor invasion and metastasis. Rhizoma Paridis saponins primarily impede cancer cell migration, invasion and metastasis by modulating MMPs and EMT, as well as regulating EGFR-mediated migration, or employing other regulation mechanisms. Created with BioRender.com, OZ27961XDJ.
5.1. MMPs and EMT
Matrix metalloproteinases (MMPs) are a part of the zinc-dependent neutral peptidase family, which collectively possess the ability to degrade components of the ECM. Initially, MMPs defined as enzymes responsible for tadpole tail dissolution, subsequent scientific research has revealed that these proteinases are vital in the ECM remodeling associated with a range of physiological processes such as uterine involution, bone resorption, and wound healing [149,150]. MMPs degrade the basement membrane and ECM, facilitating malignant cells to invade through connective tissue and blood vessel walls, leading to metastasis [151]. RPS extraction exhibits inhibitory effects on the growth of A549 cells, effectively inhibiting the adhesion, migration, and invasion behaviors. Furthermore, RPS extraction can also suppress the expression and secretion of MMP9 and MMP2, inhibiting the proliferation, migration, and differentiation of A549 cells [152]. The compounds polyphyllin II and polyphyllin E can effectively inhibit the expression of MMP2/MMP9 by downregulating the Akt/NF-κB pathway, eventually effectively suppressing the migration and invasion of cancer cells [153,154]. EMT plays an irreplaceable role throughout the entire process of cell invasion and metastasis [155]. In the process of EMT, the main changes include a decrease in the level of the epithelial marker E-cadherin (CDH1), an increase in the level of the mesenchymal marker N-cadherin (CDH2), and an increase in the levels of the Snail family transcriptional repressor 2 (SNAI2) and the Twist family BHLH transcription factor 1 (TWIST1), both of which are transcriptional inhibitors of CDH1 [156]. Polyphyllin II treatment significantly increased CDH1 expression in bladder cancer cells, and reduced the expression of CDH2, SNAI2, TWIST1, MMP2, and MMP9. These results suggest that Polyphyllin II can inhibit the invasion and migration by regulating EMT-related proteins and MMPs [153,157].
5.2. EGFR-mediated migration
EGFR, a receptor tyrosine kinase, can phosphorylate the tyrosine residues of proteins and it is a key regulatory factor in cellular processes such as cell proliferation, differentiation, survival, and migration [158,159]. PTPN11 (encoding SHP2) enhances EGFR signal transduction by dephosphorylating RasGAP, which is a negative regulatory factor in the Ras pathway. SHP2 includes two Src homology 2 (SH2) domains, N-terminal (N-SH2) and C-terminal (C-SH2), located in the PTP catalytic domain structure [160]. The N-SH2 domain negatively regulates the activity of protein tyrosine phosphatase (PTP) by interacting with the catalytic domain [161]. The growth factor receptor-bound protein 2 (GRB2) associated binding protein 1 (Gab1) binds to SHP2 and activates SHP2 by interaction with the N-SH2 domain, leading to inactivation of RasGAP and subsequent activation of Ras, further activating the Raf/MEK/ERK pathway to promote tumor cell proliferation, growth, survival, and metastasis [162]. Polyphyllin D acts as a selective SHP2 inhibitor with good efficacy, and the binding of polyphyllin D and SHP2 renders SHP2 inactive [163]. Circulating tumor cells (CTCs) are cells that are separated from the primary tumor and enter the bloodstream, playing a role between the primary and metastatic tumors [164]. Studies have shown that the protein levels of MMP2 and MMP9 can significantly reduced with treatment of polyphyllin VII in CTC-TJH-01 and lung cancer cells, effectively inhibiting the EGFR-Ras/Raf-MEK/ERK signaling pathways. In addition, Anoikis is a specific programmed cell death triggered by the loss of cell adhesion to the ECM [165]. Polyphyllin VII can induce Anoikis by downregulating the EGFR pathway and significantly reducing the risk of metastasis [166].
5.3. Other regulation mechanisms
Many scientific studies have indicated that the enhanced activity of AP-1 is mainly associated with the c-Jun subunit, which is considered as an oncogenic element, involving processes of growth, metastasis, and drug resistance [167,168]. Polyphyllin I can reduce the activity of HOTAIR, increase the protein expression of transcription factor c-Jun, stimulate the protein expression of cyclin-dependent kinase inhibitor p21 and the activity of its promoter, effectively inhibiting the growth and migration of human lung cancer cells [49,169]. In addition, polyphyllin I, polyphyllin II, polyphyllin VI, and polyphyllin VII can significantly stimulate the release of lactate dehydrogenase by human umbilical vein endothelial cells (HUVECs), resulting in inhibiting the migration and invasion. Among them, polyphyllin I shows the most significant inhibitory effect on cell invasion [170]. The endoplasmic reticulum chaperone protein GRP78 regulates cancer cells invasion and metastasis, and studies have shown that downregulation of GRP78 induced by polyphyllin I results in reduced cell migration and invasion [171]. Research has shown that polyphyllin VII can inhibit osteoclastogenesis through the c-Fos/NFATc1 signaling pathway, thereby preventing bone resorption caused by breast cancer. Polyphyllin VII can suppress osteoclast formation in bone marrow-derived macrophages (BMM) induced by breast cancer cells (MDA-MB-231 CM). Furthermore, polyphyllin VII significantly reduced osteoclast bone resorption and F-actin ring formation induced by MDA-MB-231 CM in vitro. During the process of osteoclastogenesis, polyphyllin VII markedly downregulated the activation of the c-Fos and NFATc1 signaling pathways, leading to the downregulation of osteoclast-related genes such as Oscar, Atp6v0d2, MMP9, integrin β3. However, there is one study showed opposite results that polyphyllin VII promotes osteoblastogenesis [172]. It indicates that more scientific research is needed for clarifying the mechanism clearly.
Tumor metastasis refers to the process in which tumor cells grow and settle at a distance from their primary sites, and this is one of the most lethal features of cancer. The cause of death for most cancer patients is due to metastasis rather than the primary tumor itself. The occurrence of metastasis is often accompanied by EMT, which allows cancer cells to detach from the primary tumor and spread to other sites. Therefore, inhibiting tumor invasion and metastasis is prominent for preventing the late-stage deterioration of cancer and improving patient survival rates. The comprehensive analysis shows that RPS inhibit cancer cell migration and invasion by modulating MMPs and EMT. RPS can reduce the risk of cancer cell metastasis by regulating EGFR, which can increase the protein expression of the transcription factor c-Jun, inhibit the downregulation of SHP2 and GRP78, modulate the signaling of c-Fos/NFATc1 and release lactate dehydrogenase, eventually inhibiting the process of cancer cell metastasis. Research has shown that gracillin can inhibit migration of human colorectal cancer cells by suppressing the phosphorylation pathway of STAT3 and IL-6 induced p-STAT3 nuclear translocation [173].
6. Inhibition of the generation of vasculogenic mimicry
Vasculogenic mimicry (VM) is a simulation process in which tumor cells mimic the formation of normal blood vessels to construct a network structure similar to blood vessels allowing tumors to acquire the necessary nutrients and oxygen [174]. The results showed that the ethanol extraction of RPS can inhibit VM and tumor growth in osteosarcoma by reducing the activity of migration-inducing gene 7 (MIG-7), which is considered as a key molecule in promoting VM in tumors [175]. Tumor-mediated VM is a complex process involving multiple steps, including cell adhesion, ECM remodeling, and rearrangement of actin filaments. During this process, MIG-7, a protein-rich in cysteine, is specifically overexpressed in metastatic cancers. MIG-7 promotes VM formation in osteosarcoma by activating the PI3K/MMPs/Ln-5γ2 pathway [176]. After treatment with RPS extraction, the expression levels of MIG-7, p-PI3K, p-Akt, p-mTOR, MMP2, MMP14, Ln5γ2′, and Ln5γ2x were significantly reduced. It indicates that RPS extraction effectively inhibits the signaling pathway of MIG-7/PI3K/MMPs/Ln-5γ2, by reducing the formation of lamellipodia and filopodia to prevent VM. This further confirms that RPS can inhibit the formation of VM in osteosarcoma cells by regulating the expression of MIG-7 [177]. During the formation of VM, cancer stem cells (CSC) and EMT play important roles [178]. The core transcription factor Twist1 of EMT binds to the promoter of VE-cadherin, accelerating the process of VM formation, which is disrupted by polyphyllin I through the PI3K-Akt-Twist1-VE-cadherin pathway, resulting in damage to VM. Polyphyllin I blocked the transcriptional activity of the Twist1 promoter and affected its ability to bind to the VE-cadherin promoter, leading to the inhibition of VM and exerting a dual effect on Twist1 [179] (Fig. 6).
Fig. 6.
Visualization of Rhizoma Paridis saponins inhibiting the generation of vasculogenic mimicry (VM). Rhizoma Paridis saponins effectively inhibit tumor VM through the regulation of various molecules (p-PI3K, p-Akt, MMP2, MIG-7, etc.), modulation of Twist1 and VE-cadherin expression, and inhibition of VEGFR2 activation. Created with BioRender.com, RV27962F7H.
Polyphyllin II exhibits a significant inhibitory effect on the growth of human umbilical vein endothelial cells triggered by vascular endothelial growth factor (VEGF) with dose and time dependency. In addition, polyphyllin II can interfere with the formation of renal tubules, the mechanism of which is related to the direct disruption of the glycoprotein receptors on the membrane of vascular smooth muscle cells. Polyphyllin II has been shown to effectively inhibit microvessel growth to suppress vascular formation in an ovarian cancer mouse model, furthermore, polyphyllin II can promote the proliferation of fibroblasts. In tumor cells, VEGF downregulated when treated with polyphyllin II, which led to a decrease in the phosphorylation levels of VEGF-induced vascular generation-related kinases such as extracellular signal-regulated kinase, Src family kinases, focal adhesion kinase, and Akt kinase by inhibiting the activation of VEGFR2 [180].
In conclusion, RPS can effectively inhibit the VM of tumor by regulating the activity of MIG-7, influencing the expression of Twist1 and VE-cadherin, and inhibiting the activation of VEGFR2.
7. Others
In addition to RPS impact on the occurrence and development of cancer, RPS have the ability on platelet aggregation, liver fibrosis, and immune-related signaling molecules. RPS exhibit characteristics of inducing platelet aggregation, further can increase the activity of thrombin. Thrombin as a local hemostatic drug is difficult to be obtained, and RPS have the potential to become an alteration. It was found that polyphyllin H, polyphyllin I, polyphyllin II, and polyphyllin VII all showed effective hemostatic effects in mouse tail-cutting experiments [181,182] (Fig. 7).
Fig. 7.
The effects of Rhizoma Paridis saponins on inhibiting liver fibrosis, inducing platelet aggregation, and boosting immune response. Created with BioRender.com, VJ279632CN.
Liver fibrosis is a complex process involving multiple stages triggered by various factors such as viral hepatitis, excessive alcohol consumption, biliary obstruction, and liver toxins [183]. Necrosis and regeneration of hepatocytes after liver cell injury are key steps in the process of liver fibrosis, involving mechanisms such as activation of cytokines, increased synthesis of ECM, and reduced degradation. Liver cirrhosis marks the advanced stage of liver fibrosis. Collagen fiber synthesis in the liver increases under normal circumstances, but excessive collagen deposition leads to inflammation and regeneration disorders when the liver is damaged or diseased. Liver fibrosis is reversible and closely related to the apoptosis process of activated hepatic stellate cells. Therefore, inhibiting the proliferation of activated hepatic stellate cells or promoting their regeneration may be an effective method for treating liver diseases [184]. Results showed that RPS extraction can reduce the levels of p-ERK1/2 mRNA and RASAL1 protein, and increase the concentration of p-ERK1/2 mRNA and RASAL1 protein, thereby contributing to alleviate degenerative changes and necrosis in liver tissue, reducing the risk of excessive fibrous tissue growth [185,186]. Polyphyllin D can combate drug resistance by stimulating hypertrophied lysosomes targeting acidic sphingomyelinase (SMPD1) in liver cancer. Lysosomal can be damaged when treated with polyphyllin D, such as blocking autophagic pathways, loss of lysosomal acidity, and release of lysosomal contents, thereby exhibiting anti-cancer effects on liver cancer cells in vitro and in vivo. Further research has revealed that polyphyllin D can inhibit the activity of SMPD1, which is a lysosomal acidic phosphodiesterase that catalyzes the hydrolysis of sphingomyelin by directly filling its surface groove, producing ceramide and phosphocholine [187]. Prolonged inhibition of SMPD1 activity by polyphyllin D leads to lysosomal damage and induces lysosome-dependent cell death. Moreover, polyphyllin D can also enhance lysosomal membrane permeability, release sorafenib, and enhance the anti-cancer effects of sorafenib [188].
Polyphyllin VII can activate the stimulator of interferon genes (STING) pathway through targeting macrophages, triggering the infiltration of cytotoxic T cells into lung cancer cells, initiating an immune response to combat tumors. The STING-activated innate immune pathway helps the host to resist pathogen infections and initiates effective anti-tumor adaptive immunity [189]. Macrophages play a significant role in the process of tumor infiltrating immune cells [190]. Studies have shown that STING-activated macrophages released large amounts of cytokines and chemokines, not only promoting the infiltration of cytotoxic T cells but also enhancing their cytotoxicity against lung cancer. Polyphyllin VII can guide macrophages to transform into STING-controlled M1-type macrophages, showing anti-tumor effects in the accumulation of CD8+ T cells [191].
8. Toxicity of RPS
Research has found that RPS canexhibit hepatotoxicity. Polyphyllin VI has been confirmed to have significant hepatotoxicity on HepaRG liver stem cells showing that it increased the production of ROS, prompted cytochrome c to be released from mitochondria to the cytoplasm, and activated a series of proteins (such as Fas, caspase-3, caspase-8, caspase-9, and poly (ADP-ribose) polymerase), resulting in causing morphological changes and inducing apoptosis. It is showed that the IC50 values of Polyphyllin I, Polyphyllin II, Polyphyllin VI and Polyphyllin VII were 4.666 μM, 3.747 μM, 6.656 μM and 2.75 μM in HepaRG cells, respectively. Regarding to 72 h-cytotoxicity test on HL-7702 cells, the IC50 values of Polyphyllin I, Polyphyllin II, Polyphyllin VI and Polyphyllin VII were 0.5 μM, 0.701 μM, 3.898 μM and 0.505 μM, respectively [192]. Particularly, polyphyllin I exhibits strong cytotoxicity. It is also demonstrated that polyphyllin I has the ability to directly bind with SQLE protein, leading to significant disruption in lipid metabolism through the SREBP-2/HMGCR/SQLE/LSS pathway, potentially causing liver toxicity [193]. Also, animal studies have shown that RPS extraction have side effects such as nausea, vomiting, diarrhea [194], and polyphyllin D can even cause hemolysis [195].
It has been reported that the water extract of Curcuma (CW) can significantly enhance the anti-cancer effects and reduce harmful effects of RPS, such as restoring liver function and improving functional indicators, while normalizing abnormal areas in the liver and lungs. CW demonstrated an inhibitory effect on the proliferation of liver cancer cells in mice, with no significant toxic side effects observed. CW may contribute to reducing RPS toxicity by promoting the binding of Trx and TXNIP, enhancing the activity of HO-1, GSH, SOD, and Nrf2, while lowering levels of ROS, MDA, and 8-OHdG, as well as decreasing the expression of COX-2, IL-1B, and NF-kB. The ideal ratio of curcuminoids in CW to RPS is 16:500 (w/w). Additionally, compounds such as curcuminoids reduced the promoting effect of RPS on the efflux of Rhodamine 123. The function of curcuminoids is similar to that of P-glycoprotein (P-gp) inhibitors, which means that the combination of cyclosporin A and RPS exhibits comparable effects [196]. The LouHuang preparation (LH), a combination of Rhizoma Curcuma longa and RPS, has been shown to enhance the anti-cancer effects of RPS while reducing its toxicity. Acute oral toxicity testing determined the LD50 of LH in mice was 3410.9 mg/kg. LH significantly mitigated the inhibitory effect of RPS on gastric emptying and inhibited tumor growth compared to RPS alone [197].
9. Conclusions
Chinese herbal medicine as an alternative treatment for cancer has demonstrated its powerful potentials, providing a new and complementary method for cancer therapy. The combination of chemotherapy and Chinese herbal medicine (CHM) has gained attention for its potential to enhance treatment outcomes in cancer care such as improving the prognosis of cancer patients and reducing the side effects. Rhizoma Paridis, a documented Chinese herbal medicine in ancient times, contains the main anti-tumor active ingredient RPS, which could be new drug candidates for cancer therapy based on the deep research on the pharmacology and toxicology in recent years (Fig. 8). The review comprehensively summaries the current research progress on RPS to provide a basic literature reference for scientific research. It is still a long way to go before RPS formally enters clinical application. Extensive research is necessary to explore and gain an in-depth understanding of underlying molecular mechanisms in the future.
Fig. 8.
A comprehensive overview of the molecular targets of Rhizoma Paridis saponins in anti-cancer activity. Created with BioRender.com, PW27962RRU.
Data availability statement
Data availability is not applicable to this article as no new data were created or analyzed in this study.
Funding
The publication of this article was funded by Southwest Medical University (05/00170050), and Sichuan Science and Technology Program (Grant No. 2022YFS0623).
Ethics statement
Review and/or approval by an ethics committee was not needed because this article did not involve human or animal studies.
CRediT authorship contribution statement
Famin Ke: Writing – original draft. Ranqi Zhang: Writing – original draft. Rui Chen: Writing – review & editing. Xiurong Guo: Writing – review & editing. Can Song: Writing – review & editing. Xiaowei Gao: Writing – review & editing. Fancai Zeng: Conceptualization. Qiuyu Liu: Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Fancai Zeng, Email: zfcai@swmu.edu.cn.
Qiuyu Liu, Email: q.liu@swmu.edu.cn.
References
- 1.Song G., Cheng L., Chao Y., Yang K., Liu Z. Emerging nanotechnology and advanced materials for cancer radiation therapy. Adv. Mater. 2017;29 doi: 10.1002/adma.201700996. [DOI] [PubMed] [Google Scholar]
- 2.Chen W., Zheng R., Zuo T., Zeng H., Zhang S., He J. National cancer incidence and mortality in China, 2012. Chin. J. Cancer Res. 2016;28:1–11. doi: 10.3978/j.issn.1000-9604.2016.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liao Z., Tan Z.W., Zhu P., Tan N.S. Cancer-associated fibroblasts in tumor microenvironment – accomplices in tumor malignancy. Cell. Immunol. 2019;343 doi: 10.1016/j.cellimm.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Bray F., Laversanne M., Sung H., Ferlay J., Siegel R.L., Soerjomataram I., Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024;74:229–263. doi: 10.3322/caac.21834. [DOI] [PubMed] [Google Scholar]
- 5.Zheng R.S., Chen R., Han B.F., Wang S.M., Li L., Sun K.X., Zeng H.M., Wei W.W., He J. [Cancer incidence and mortality in China, 2022] Zhonghua Zhongliu Zazhi. 2024;46:221–231. doi: 10.3760/cma.j.cn112152-20240119-00035. [DOI] [PubMed] [Google Scholar]
- 6.Chang Y., He L., Li Z., Zeng L., Song Z., Li P., Chan L., You Y., Yu X.-F., Chu P.K., Chen T. Designing core–shell gold and selenium nanocomposites for cancer radiochemotherapy. ACS Nano. 2017;11:4848–4858. doi: 10.1021/acsnano.7b01346. [DOI] [PubMed] [Google Scholar]
- 7.Schirrmacher V. From chemotherapy to biological therapy: a review of novel concepts to reduce the side effects of systemic cancer treatment. Int. J. Oncol. 2018;54:407–419. doi: 10.3892/ijo.2018.4661. (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X., Qiu H., Li C., Cai P., Qi F. The positive role of traditional Chinese medicine as an adjunctive therapy for cancer. BST. 2021;15:283–298. doi: 10.5582/bst.2021.01318. [DOI] [PubMed] [Google Scholar]
- 9.Xiang Y., Guo Z., Zhu P., Chen J., Huang Y. Traditional Chinese medicine as a cancer treatment: modern perspectives of ancient but advanced science. Cancer Med. 2019;8:1958–1975. doi: 10.1002/cam4.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.X. Guan, R. Li, B. Duan, Y. Wang, M. Fan, S. Wang, H. Zhang, C. Xia, Advances in research on chemical constituents and pharmacological effects of Paris genus and prediction and analysis of quality markers, Chin. Tradit. Herb. Drugs 50 (n.d.) 4838–4852. 10.7501/j.issn.0253-2670.2019.19.034. . [DOI]
- 11.Wu X., Wang L., Wang G.-C., Wang H., Dai Y., Ye W.-C., Li Y.-L. New steroidal saponins and sterol glycosides from Paris polyphylla var. yunnanensis. Planta Med. 2012;78:1667–1675. doi: 10.1055/s-0032-1315239. [DOI] [PubMed] [Google Scholar]
- 12.Zhou N., Xu L., Park S.-M., Ma M.-G., Choi S.-E., Si C. Genetic diversity, chemical components, and property of biomass Paris polyphylla var. yunnanensis. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.713860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y., Gu J.-F., Zou X., Wu J., Zhang M.-H., Jiang J., Qin D., Zhou J.-Y., Liu B.-X.-Z., Zhu Y.-T., Jia X.-B., Feng L., Wang R.-P. The anti-lung cancer activities of steroidal saponins of P. Polyphylla smith var. chinensis (Franch.) Hara through enhanced immunostimulation in experimental lewis tumor-bearing C57BL/6 mice and induction of apoptosis in the A549 cell line. Molecules. 2013;18:12916–12936. doi: 10.3390/molecules181012916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y., Liu M.-Y., Bi L.-L., Tian Y.-Y., Qiu P.-C., Qian X.-Y., Wang M.-C., Tang H.-F., Lu Y.-Y., Zhang B.-L. Cytotoxic steroidal glycosides from the rhizomes of Paris polyphylla var. yunnanensis. Phytochemistry. 2023;207 doi: 10.1016/j.phytochem.2022.113577. [DOI] [PubMed] [Google Scholar]
- 15.Wu X., Wang L., Wang G.-C., Wang H., Dai Y., Yang X.-X., Ye W.-C., Li Y.-L. Triterpenoid saponins from rhizomes of Paris polyphylla var. yunnanensis. Carbohydr. Res. 2013;368:1–7. doi: 10.1016/j.carres.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 16.L. zhou, Heptasaccharide and Octasaccharide Isolated from Paris polyphylla Var. Yunnanensis and Their Plant Growth-Regulatory Activity, (n.d.). .
- 17.Zhang J.-Y., Wang Y.-Z., Zhao Y.-L., Yang S.-B., Zuo Z.-T., Yang M.-Q., Zhang J., Yang W.-Z., Yang T.-M., Jin H. Phytochemicals and bioactivities of Paris species. J. Asian Nat. Prod. Res. 2011;13:670–681. doi: 10.1080/10286020.2011.578247. [DOI] [Google Scholar]
- 18.Jing S., Wang Y., Li X., Man S., Gao W. Chemical constituents and antitumor activity from Paris polyphylla Smith var. yunnanensis. Nat. Prod. Res. 2017;31:660–666. doi: 10.1080/14786419.2016.1219861. [DOI] [PubMed] [Google Scholar]
- 19.Shen S., Xu Z., Feng S., Wang H., Liu J., Zhou L., Yuan M., Huang Y., Ding C. Structural elucidation and antiaging activity of polysaccharide from Paris polyphylla leaves. Int. J. Biol. Macromol. 2018;107:1613–1619. doi: 10.1016/j.ijbiomac.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Qin X.-J., Sun D.-J., Ni W., Chen C.-X., Hua Y., He L., Liu H.-Y. Steroidal saponins with antimicrobial activity from stems and leaves of Paris polyphylla var. yunnanensis. Steroids. 2012;77:1242–1248. doi: 10.1016/j.steroids.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Wei J., Gao W., Yan X., Wang Y., Jing S., Xiao P. ChemInform abstract: chemical constituents of plants from the genus paris. ChemInform. 2014;45 doi: 10.1002/chin.201450224. chin. [DOI] [PubMed] [Google Scholar]
- 22.Wang G.-X., Han J., Zhao L.-W., Jiang D.-X., Liu Y.-T., Liu X.-L. Anthelmintic activity of steroidal saponins from Paris polyphylla. Phytomedicine. 2010;17:1102–1105. doi: 10.1016/j.phymed.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 23.Yue J., Li Z., Zuo Z., Zhao Y., Zhang J., Wang Y. Study on the identification and evaluation of growth years for Paris polyphylla var. yunnanensis using deep learning combined with 2DCOS. Spectrochim. Acta Mol. Biomol. Spectrosc. 2021;261 doi: 10.1016/j.saa.2021.120033. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X., Cui Y., Huang J., Zhang Y., Nie Z., Wang L., Yan B., Tang Y., Liu Y. Immuno-stimulating properties of diosgenyl saponins isolated from Paris polyphylla. Bioorg. Med. Chem. Lett. 2007;17:2408–2413. doi: 10.1016/j.bmcl.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 25.Deng D., Lauren D., Cooney J., Jensen D., Wurms K., Upritchard J., Cannon R., Wang M., Li M. Antifungal saponins from Paris polyphylla smith. Planta Med. 2008;74:1397–1402. doi: 10.1055/s-2008-1081345. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y., Jiang Y., Yang C., Wang J., Xu Z., Liu Y., Duan B. Research progress on chemical constituents, pharmacological actities, and clinical applications of Paris polyphylla var. yunnanensis. Chin. Tradit. Herb. Drugs. 2022;53:7633–7648. doi: 10.7501/j.issn.0253-2670.2022.23.035. [DOI] [Google Scholar]
- 27.Kang L.-P., Liu Y.-X., Eichhorn T., Dapat E., Yu H., Zhao Y., Xiong C.-Q., Liu C., Efferth T., Ma B.-P. Polyhydroxylated steroidal glycosides from Paris polyphylla. J. Nat. Prod. 2012;75:1201–1205. doi: 10.1021/np300045g. [DOI] [PubMed] [Google Scholar]
- 28.Qin X.-J., Ni W., Chen C.-X., Liu H.-Y. Seeing the light: shifting from wild rhizomes to extraction of active ingredients from above-ground parts of Paris polyphylla var. yunnanensis. J. Ethnopharmacol. 2018;224:134–139. doi: 10.1016/j.jep.2018.05.028. [DOI] [PubMed] [Google Scholar]
- 29.Wu X., Wang L., Wang H., Dai Y., Ye W.-C., Li Y.-L. Steroidal saponins from Paris polyphylla var. yunnanensis. Phytochemistry. 2012;81:133–143. doi: 10.1016/j.phytochem.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 30.Liu J., Zhang Y., Chen L., Yu F., Li X., Tao Dan, Zhao J., Zhou S. Polyphyllin I induces G2/M phase arrest and apoptosis in U251 human glioma cells via mitochondrial dysfunction and the JNK signaling pathway. Acta Biochim. Biophys. Sin. 2017;49:479–486. doi: 10.1093/abbs/gmx033. [DOI] [PubMed] [Google Scholar]
- 31.He J., Yu S., Guo C., Tan L., Song X., Wang M., Wu J., Long Y., Gong D., Zhang R., Cao Z., Li Y., Peng C. Polyphyllin I induces autophagy and cell cycle arrest via inhibiting PDK1/Akt/mTOR signal and downregulating cyclin B1 in human gastric carcinoma HGC-27 cells. Biomed. Pharmacother. 2019;117 doi: 10.1016/j.biopha.2019.109189. [DOI] [PubMed] [Google Scholar]
- 32.Shuli M., Wenyuan G., Yanjun Z., Chaoyi M., Liu Y., Yiwen L. Paridis saponins inhibiting carcinoma growth and metastasis in vitro and in vivo. Arch Pharm. Res. (Seoul) 2011;34:43–50. doi: 10.1007/s12272-011-0105-4. [DOI] [PubMed] [Google Scholar]
- 33.Sun J., Liu B., Hu W., Yu L., Qian X. In vitro anticancer activity of aqueous extracts and ethanol extracts of fifteen taditional Chinese medicines on human digestive tumor cell lines. Phytother Res. 2007;21:1102–1104. doi: 10.1002/ptr.2196. [DOI] [PubMed] [Google Scholar]
- 34.Qin X.-J., Yu M.-Y., Ni W., Yan H., Chen C.-X., Cheng Y.-C., He L., Liu H.-Y. Steroidal saponins from stems and leaves of Paris polyphylla var. yunnanensis. Phytochemistry. 2016;121:20–29. doi: 10.1016/j.phytochem.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Kong M., Fan J., Dong A., Cheng H., Xu R. Effects of polyphyllin I on growth inhibition of human non-small lung cancer cells and in xenograft. ABBS. 2010;42:827–833. doi: 10.1093/abbs/gmq091. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y., Huang P., Liu X., Xiang Y., Zhang T., Wu Y., Xu J., Sun Z., Zhen W., Zhang L., Si Y., Liu Y. Polyphyllin I inhibits growth and invasion of cisplatin-resistant gastric cancer cells by partially inhibiting CIP2A/PP2A/Akt signaling axis. J. Pharmacol. Sci. 2018;137:305–312. doi: 10.1016/j.jphs.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Teng W.-J., Chen P., Zhu F.-Y., Di K., Zhou C., Zhuang J., Cao X.-J., Yang J., Deng L.-J., Sun C.-G. Effect of Rhizoma paridis total saponins on apoptosis of colorectal cancer cells and imbalance of the JAK/STAT3 molecular pathway induced by IL-6 suppression. Genet. Mol. Res. 2015;14:5793–5803. doi: 10.4238/2015.May.29.11. [DOI] [PubMed] [Google Scholar]
- 38.Man S., Gao W., Yan Y., Liu Z., Liu C. Inhibition of matrix metalloproteinases related to metastasis by diosgenyl and pennogenyl saponins. J. Ethnopharmacol. 2011;137:1221–1227. doi: 10.1016/j.jep.2011.07.045. [DOI] [PubMed] [Google Scholar]
- 39.Tian Y., Gong G.-Y., Ma L.-L., Wang Z.-Q., Song D., Fang M.-Y. Anti-cancer effects of Polyphyllin I: an update in 5 years. Chem. Biol. Interact. 2020;316 doi: 10.1016/j.cbi.2019.108936. [DOI] [PubMed] [Google Scholar]
- 40.Lin R.-K., Wang Y.-C. Dysregulated transcriptional and post-translational control of DNA methyltransferases in cancer. Cell Biosci. 2014;4:46. doi: 10.1186/2045-3701-4-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu P., Man S., Yang H., Fan W., Yu P., Gao W. Utilization of metabonomics to identify serum biomarkers in murine H22 hepatocarcinoma and deduce antitumor mechanism of Rhizoma Paridis saponins. Chem. Biol. Interact. 2016;256:55–63. doi: 10.1016/j.cbi.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 42.Egbuna C., Patrick‐Iwuanyanwu K.C., Onyeike E.N., Uche C.Z., Ogoke U.P., Riaz M., Ibezim E.N., Khan J., Adedokun K.A., Imodoye S.O., Bello I.O., Awuchi C.G. Wnt/β‐catenin signaling pathway inhibitors, glycyrrhizic acid, solanine, polyphyllin I, crocin, hypericin, tubeimoside‐1, diosmin, and rutin in medicinal plants have better binding affinities and anticancer properties: molecular docking and ADMET study. Food Sci. Nutr. 2023;11:4155–4169. doi: 10.1002/fsn3.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo Y., Pan W., Liu S., Shen Z., Xu Y., Hu L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020 doi: 10.3892/etm.2020.8454. (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang G., Niu Y., Li Y., Wu C., Wang X., Zhang J. Paris saponin VII enhanced the sensitivity of HepG2/ADR cells to ADR via modulation of PI3K/AKT/MAPK signaling pathway. Kaohsiung J. Med. Sci. 2020;36:98–106. doi: 10.1002/kjm2.12145. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Bieging K.T., Mello S.S., Attardi L.D. Unravelling mechanisms of p53-mediated tumour suppression. Nat. Rev. Cancer. 2014;14:359–370. doi: 10.1038/nrc3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muller P.A.J., Vousden K.H. p53 mutations in cancer. Nat. Cell Biol. 2013;15:2–8. doi: 10.1038/ncb2641. [DOI] [PubMed] [Google Scholar]
- 47.Abbas T., Dutta A. p21 in cancer: intricate networks and multiple activities. Nat. Rev. Cancer. 2009;9:400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gartel A.L., Serfas M.S., Tyner A.L. p21--Negative regulator of the cell cycle. Exp. Biol. Med. 1996;213:138–149. doi: 10.3181/00379727-213-44046. [DOI] [PubMed] [Google Scholar]
- 49.Zhao Y., Tang X., Huang Y., Tang Q., Ma C., Zheng F., Wu W., Hann S.S. Interaction of c-jun and HOTAIR- increased expression of p21 Converge in polyphyllin I-inhibited growth of human lung cancer cells. OTT. 2019;12:10115–10127. doi: 10.2147/OTT.S226830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen Z., Wang J., Ke K., Chen R., Zuo A., Zhang R., Wan W., Xie X., Li X., Song N., Fu H., Zhang Z., Cai E., Shen J., Zhang Q., Shi X., Polyphyllin I. A lethal partner of Palbociclib, suppresses non-small cell lung cancer through activation of p21/CDK2/Rb pathway in vitro and in vivo. Cell Cycle. 2021;20:2494–2506. doi: 10.1080/15384101.2021.1991121. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Zhang D., Liu S., Liu Z., Ma C., Jiang Y., Sun C., Li K., Cao G., Lin Z., Wang P., Zhang J., Xu D., Kong F., Zhao S. Polyphyllin I induces cell cycle arrest in prostate cancer cells via the upregulation of IL6 and P21 expression. Medicine. 2019;98 doi: 10.1097/MD.0000000000017743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li H.S., Xu Y. Inhibition of EZH2 via the STAT3/HOTAIR signalling axis contributes to cell cycle arrest and apoptosis induced by polyphyllin I in human non-small cell lung cancer cells. Steroids. 2020;164 doi: 10.1016/j.steroids.2020.108729. [DOI] [PubMed] [Google Scholar]
- 53.Zeng Y., Zhang Z., Wang W., You L., Dong X., Yin X., Qu C., Ni J. Underlying mechanisms of apoptosis in HepG2 cells induced by polyphyllin I through Fas death and mitochondrial pathways. Toxicol. Mech. Methods. 2020;30:397–406. doi: 10.1080/15376516.2020.1747125. [DOI] [PubMed] [Google Scholar]
- 54.Yu S., Wang L., Cao Z., Gong D., Liang Q., Chen H., Fu H., Wang W., Tang X., Xie Z., He Y., Peng C., Li Y. Anticancer effect of Polyphyllin Ι in colorectal cancer cells through ROS-dependent autophagy and G2/M arrest mechanisms. Nat. Prod. Res. 2018;32:1489–1492. doi: 10.1080/14786419.2017.1353512. [DOI] [PubMed] [Google Scholar]
- 55.Sawah E.A., Marchion D.C., Xiong Y., Ramirez I.J., Abbasi F., Boac B.M., Bush S.H., Zgheib N.B., McClung E.C., Khulpateea B.R., Berry A., Hakam A., Wenham R.M., Lancaster J.M., Judson P.L. The Chinese herb polyphyllin D sensitizes ovarian cancer cells to cisplatin-induced growth arrest. J. Cancer Res. Clin. Oncol. 2015;141:237–242. doi: 10.1007/s00432-014-1797-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen Y.Y., Ren C.F., Wen S.Y. Polyphyllin D induces G2/M cell cycle arrest via dysfunction of cholesterol biosynthesis in liver cancer cells. Biomed. Environ. Sci. 2023;36:94–98. doi: 10.3967/bes2023.009. [DOI] [PubMed] [Google Scholar]
- 57.Wang H., Fei Z., Jiang H. Polyphyllin VII increases sensitivity to gefitinib by modulating the elevation of P21 in acquired gefitinib resistant non-small cell lung cancer. J. Pharmacol. Sci. 2017;134:190–196. doi: 10.1016/j.jphs.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 58.Li J.-K., Sun H.-T., Jiang X.-L., Chen Y.-F., Zhang Z., Wang Y., Chen W.-Q., Zhang Z., Sze S.C.W., Zhu P.-L., Yung K.K.L. Polyphyllin II induces protective autophagy and apoptosis via inhibiting PI3K/AKT/mTOR and STAT3 signaling in colorectal cancer cells. Indian J. Manag. Sci. 2022;23 doi: 10.3390/ijms231911890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bi L., Liu Y., Yang Q., Zhou X., Li H., Liu Y., Li J., Lu Y., Tang H. Paris saponin H inhibits the proliferation of glioma cells through the A1 and A3 adenosine receptor-mediated pathway. Int. J. Mol. Med. 2021;47:30. doi: 10.3892/ijmm.2021.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang J., Cao L., Li Y., Liu H., Zhang M., Ma H., Wang B., Yuan X., Liu Q. Gracillin isolated from reineckia carnea induces apoptosis of A549 cells via the mitochondrial pathway. DDDT. 2021;15:233–243. doi: 10.2147/DDDT.S278975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu W., Wang Y., Chen J., Lin Z., Lin M., Lin X., Fan Y. Beneficial effects of gracillin from rhizoma paridis against gastric carcinoma via the potential TIPE2-mediated induction of endogenous apoptosis and inhibition of migration in BGC823 cells. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.669199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang J., Yang Y., Lei L., Tian M. Rhizoma paridis saponins induces cell cycle arrest and apoptosis in non-small cell lung carcinoma A549 cells. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2015;21:2535–2541. doi: 10.12659/MSM.895084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan S., Tian S., Kang Q., Xia Y., Li C., Chen Q., Zhang S., Li Z. Rhizoma paridis saponins suppresses tumor growth in a rat model of N-Nitrosomethylbenzylamine-Induced esophageal cancer by inhibiting cyclooxygenases-2 pathway. PLoS One. 2015;10 doi: 10.1371/journal.pone.0131560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Man S., Fan W., Liu Z., Gao W., Li Y., Zhang L., Liu C. Antitumor pathway of Rhizoma Paridis Saponins based on the metabolic regulatory network alterations in H22 hepatocarcinoma mice. Steroids. 2014;84:17–21. doi: 10.1016/j.steroids.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 65.Min H.-Y., Pei H., Hyun S.Y., Boo H.-J., Jang H.-J., Cho J., Kim J.H., Son J., Lee H.-Y. Potent anticancer effect of the natural steroidal saponin gracillin is produced by inhibiting glycolysis and oxidative phosphorylation-mediated bioenergetics. Cancers. 2020;12:913. doi: 10.3390/cancers12040913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ibrahim J., Peeters M., Van Camp G., Op De Beeck K. Methylation biomarkers for early cancer detection and diagnosis: current and future perspectives. Eur. J. Cancer. 2023;178:91–113. doi: 10.1016/j.ejca.2022.10.015. [DOI] [PubMed] [Google Scholar]
- 67.Loaeza-Loaeza J., Beltran A.S., Hernández-Sotelo D. DNMTs and impact of CpG content, transcription factors, consensus motifs, lncRNAs, and histone marks on DNA methylation. Genes. 2020;11:1336. doi: 10.3390/genes11111336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hervouet E., Vallette F.M., Cartron P.-F. Impact of the DNA methyltransferases expression on the methylation status of apoptosis-associated genes in glioblastoma multiforme. Cell Death Dis. 2010;1:e8. doi: 10.1038/cddis.2009.7. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li L., Wu J., Zheng F., Tang Q., Wu W., Hann S.S. Inhibition of EZH2 via activation of SAPK/JNK and reduction of p65 and DNMT1 as a novel mechanism in inhibition of human lung cancer cells by polyphyllin I. J. Exp. Clin. Cancer Res. 2016;35:112. doi: 10.1186/s13046-016-0388-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zou P.-L., Zhang Q.-H., Zhou J.-F., Lin R.-W., Chen Z.-Q., Xiang S.-T. [Inhibitory effect of polyphyllin Ⅰ on the proliferation of prostate cancer PC3 cells via ERK1/2/P65/DNMT1 and its molecular mechanism] Zhonghua Nan ke Xue. 2018;24:199–205. [PubMed] [Google Scholar]
- 71.Fan C.-H., Liu W.-L., Cao H., Wen C., Chen L., Jiang G. O6-methylguanine DNA methyltransferase as a promising target for the treatment of temozolomide-resistant gliomas. Cell Death Dis. 2013;4:e876. doi: 10.1038/cddis.2013.388. e876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Margison G.P. Mechanisms of carcinogenicity/chemotherapy by O6-methylguanine. Mutagenesis. 2002;17:483–487. doi: 10.1093/mutage/17.6.483. [DOI] [PubMed] [Google Scholar]
- 73.Pang D., Li C., Yang C., Zou Y., Feng B., Li L., Liu W., Geng Y., Luo Q., Chen Z., Huang C. Polyphyllin VII promotes apoptosis and autophagic cell death via ROS-inhibited AKT activity, and sensitizes glioma cells to temozolomide. Oxid. Med. Cell. Longev. 2019;2019:1–19. doi: 10.1155/2019/1805635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu Q., Krause M., Samoylenko A., Vainio S. Wnt signaling in renal cell carcinoma. Cancers. 2016;8:57. doi: 10.3390/cancers8060057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bullions L.C., Levine A.J. The role of beta-catenin in cell adhesion, signal transduction, and cancer. Curr. Opin. Oncol. 1998;10:81–87. doi: 10.1097/00001622-199801000-00013. [DOI] [PubMed] [Google Scholar]
- 76.Gordon M.D., Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J. Biol. Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 77.Bienz M. The subcellular destinations of apc proteins. Nat. Rev. Mol. Cell Biol. 2002;3:328–338. doi: 10.1038/nrm806. [DOI] [PubMed] [Google Scholar]
- 78.Stamos J.L., Weis W.I. The -catenin destruction complex. Cold Spring Harbor Perspect. Biol. 2013;5 doi: 10.1101/cshperspect.a007898. a007898–a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang H., Xiao X., Li Z., Luo S., Hu L., Yi H., Xiang R., Zhu Y., Wang Y., Zhu L., Xiao L., Dai C., Aziz A., Yuan L., Cui Y., Li R., Gong F., Liu X., Liang L., Peng H., Zhou H., Liu J. Polyphyllin VII, a novel moesin inhibitor, suppresses cell growth and overcomes bortezomib resistance in multiple myeloma. Cancer Lett. 2022;537 doi: 10.1016/j.canlet.2022.215647. [DOI] [PubMed] [Google Scholar]
- 80.Galluzzi L., Vitale I., Aaronson S.A., Abrams J.M., Adam D., Agostinis P., Alnemri E.S., Altucci L., Amelio I., Andrews D.W., Annicchiarico-Petruzzelli M., Antonov A.V., Arama E., Baehrecke E.H., Barlev N.A., Bazan N.G., Bernassola F., Bertrand M.J.M., Bianchi K., Blagosklonny M.V., Blomgren K., Borner C., Boya P., Brenner C., Campanella M., Candi E., Carmona-Gutierrez D., Cecconi F., Chan F.K.-M., Chandel N.S., Cheng E.H., Chipuk J.E., Cidlowski J.A., Ciechanover A., Cohen G.M., Conrad M., Cubillos-Ruiz J.R., Czabotar P.E., D'Angiolella V., Dawson T.M., Dawson V.L., De Laurenzi V., De Maria R., Debatin K.-M., DeBerardinis R.J., Deshmukh M., Di Daniele N., Di Virgilio F., Dixit V.M., Dixon S.J., Duckett C.S., Dynlacht B.D., El-Deiry W.S., Elrod J.W., Fimia G.M., Fulda S., García-Sáez A.J., Garg A.D., Garrido C., Gavathiotis E., Golstein P., Gottlieb E., Green D.R., Greene L.A., Gronemeyer H., Gross A., Hajnoczky G., Hardwick J.M., Harris I.S., Hengartner M.O., Hetz C., Ichijo H., Jäättelä M., Joseph B., Jost P.J., Juin P.P., Kaiser W.J., Karin M., Kaufmann T., Kepp O., Kimchi A., Kitsis R.N., Klionsky D.J., Knight R.A., Kumar S., Lee S.W., Lemasters J.J., Levine B., Linkermann A., Lipton S.A., Lockshin R.A., López-Otín C., Lowe S.W., Luedde T., Lugli E., MacFarlane M., Madeo F., Malewicz M., Malorni W., Manic G., Marine J.-C., Martin S.J., Martinou J.-C., Medema J.P., Mehlen P., Meier P., Melino S., Miao E.A., Molkentin J.D., Moll U.M., Muñoz-Pinedo C., Nagata S., Nuñez G., Oberst A., Oren M., Overholtzer M., Pagano M., Panaretakis T., Pasparakis M., Penninger J.M., Pereira D.M., Pervaiz S., Peter M.E., Piacentini M., Pinton P., Prehn J.H.M., Puthalakath H., Rabinovich G.A., Rehm M., Rizzuto R., Rodrigues C.M.P., Rubinsztein D.C., Rudel T., Ryan K.M., Sayan E., Scorrano L., Shao F., Shi Y., Silke J., Simon H.-U., Sistigu A., Stockwell B.R., Strasser A., Szabadkai G., Tait S.W.G., Tang D., Tavernarakis N., Thorburn A., Tsujimoto Y., Turk B., Vanden Berghe T., Vandenabeele P., Vander Heiden M.G., Villunger A., Virgin H.W., Vousden K.H., Vucic D., Wagner E.F., Walczak H., Wallach D., Wang Y., Wells J.A., Wood W., Yuan J., Zakeri Z., Zhivotovsky B., Zitvogel L., Melino G., Kroemer G. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peng F., Liao M., Qin R., Zhu S., Peng C., Fu L., Chen Y., Han B. Regulated cell death (RCD) in cancer: key pathways and targeted therapies. Signal Transduct. Targeted Ther. 2022;7:286. doi: 10.1038/s41392-022-01110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Christgen S., Tweedell R.E., Kanneganti T.-D. Programming inflammatory cell death for therapy. Pharmacol. Therapeut. 2022;232 doi: 10.1016/j.pharmthera.2021.108010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liao M., Qin R., Huang W., Zhu H.-P., Peng F., Han B., Liu B. Targeting regulated cell death (RCD) with small-molecule compounds in triple-negative breast cancer: a revisited perspective from molecular mechanisms to targeted therapies. J. Hematol. Oncol. 2022;15:44. doi: 10.1186/s13045-022-01260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fairlie W.D., Tran S., Lee E.F. International Review of Cell and Molecular Biology. Elsevier; 2020. Crosstalk between apoptosis and autophagy signaling pathways; pp. 115–158. [DOI] [PubMed] [Google Scholar]
- 85.Gogvadze V., Orrenius S., Zhivotovsky B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim. Biophys. Acta Bioenerg. 2006;1757:639–647. doi: 10.1016/j.bbabio.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 86.Hong F., Gu W., Jiang J., Liu X., Jiang H. Anticancer activity of polyphyllin I in nasopharyngeal carcinoma by modulation of lncRNA ROR and P53 signalling. J. Drug Target. 2019;27:806–811. doi: 10.1080/1061186X.2018.1561887. [DOI] [PubMed] [Google Scholar]
- 87.Jiang H., Zhao P.-J., Su D., Feng J., Ma S.-L. Paris saponin I induces apoptosis via increasing the Bax/Bcl-2 ratio and caspase-3 expression in gefitinib-resistant non-small cell lung cancer in vitro and in vivo. Mol. Med. Rep. 2014;9:2265–2272. doi: 10.3892/mmr.2014.2108. [DOI] [PubMed] [Google Scholar]
- 88.Chen Y.-S., He Y., Chen C., Zeng Y., Xue D., Wen F.-Y., Wang L., Zhang H., Du J.-R. Growth inhibition by pennogenyl saponins from Rhizoma paridis on hepatoma xenografts in nude mice. Steroids. 2014;83:39–44. doi: 10.1016/j.steroids.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 89.Xiao X. Paris Saponin II of Rhizoma Paridis – a novel inducer of apoptosis in human ovarian cancer cells. Biosci Trends. 2012;6:201–211. doi: 10.5582/bst.2012.v6.4.201. [DOI] [PubMed] [Google Scholar]
- 90.Jiao Y., Xin M., Xu J., Xiang X., Li X., Jiang J., Jia X. Polyphyllin II induced apoptosis of NSCLC cells by inhibiting autophagy through the mTOR pathway. Pharmaceut. Biol. 2022;60:1781–1789. doi: 10.1080/13880209.2022.2120021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen C.-R., Zhang J., Wu K.-W., Liu P.-Y., Wang S.-J., Chen D.-Y., Ji Z.-N. Gracillin induces apoptosis in HL60 human leukemic cell line via oxidative stress and cell cycle arrest of G1. Pharmazie. 2015;70:199–204. [PubMed] [Google Scholar]
- 92.Min H.-Y., Jang H.-J., Park K.H., Hyun S.Y., Park S.J., Kim J.H., Son J., Kang S.S., Lee H.-Y. The natural compound gracillin exerts potent antitumor activity by targeting mitochondrial complex II. Cell Death Dis. 2019;10:810. doi: 10.1038/s41419-019-2041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang C., Cai H., Meng X. Polyphyllin D induces apoptosis and differentiation in K562 human leukemia cells. Int. Immunopharm. 2016;36:17–22. doi: 10.1016/j.intimp.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 94.Chen J.-C., Hsieh M.-J., Chen C.-J., Lin J.-T., Lo Y.-S., Chuang Y.-C., Chien S.-Y., Chen M.-K. Polyphyllin G induce apoptosis and autophagy in human nasopharyngeal cancer cells by modulation of AKT and mitogen-activated protein kinase pathways in vitro and in vivo. Oncotarget. 2016;7:70276–70289. doi: 10.18632/oncotarget.11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu Y., Dong X., Wang W., You L., Yin X., Yang C., Sai N., Leng X., Ni J. Molecular mechanisms of apoptosis in HepaRG cell line induced by polyphyllin VI via the Fas death pathway and mitochondrial-dependent pathway. Toxins. 2018;10:201. doi: 10.3390/toxins10050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Teng J.-F., Mei Q.-B., Zhou X.-G., Tang Y., Xiong R., Qiu W.-Q., Pan R., Law B.Y.-K., Wong V.K.-W., Yu C.-L., Long H.-A., Xiao X.-L., Zhang F., Wu J.-M., Qin D.-L., Wu A.-G. Polyphyllin VI induces caspase-1-mediated pyroptosis via the induction of ROS/NF-κB/NLRP3/GSDMD signal Axis in non-small cell lung cancer. Cancers. 2020;12:193. doi: 10.3390/cancers12010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang B., Lang X., Li X. The role of IL-6/JAK2/STAT3 signaling pathway in cancers. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.1023177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Han J., Kan J., Wang X., Yuan Q., Song X., Huang M., Wu H. Polyphyllin I suppresses proliferation and promotes apoptosis of gastric cancer cell by inhibiting stat3 phosphorylation. Transl Cancer Res TCR. 2020;9:4715–4725. doi: 10.21037/tcr-20-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang Q., Chen W., Xu Y., Lv X., Zhang M., Jiang H. Polyphyllin I modulates MALAT1/STAT3 signaling to induce apoptosis in gefitinib-resistant non-small cell lung cancer. Toxicol. Appl. Pharmacol. 2018;356:1–7. doi: 10.1016/j.taap.2018.07.031. [DOI] [PubMed] [Google Scholar]
- 100.Liu W., Chai Y., Hu L., Wang J., Pan X., Yuan H., Zhao Z., Song Y., Zhang Y. Polyphyllin VI induces apoptosis and autophagy via reactive oxygen species mediated JNK and P38 activation in glioma. OTT. 2020;13:2275–2288. doi: 10.2147/OTT.S243953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang D.-W., Shao J., Lin J., Zhang N., Lu B.-J., Lin S.-C., Dong M.-Q., Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 102.Christofferson D.E., Yuan J. Necroptosis as an alternative form of programmed cell death. Curr. Opin. Cell Biol. 2010;22:263–268. doi: 10.1016/j.ceb.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Najafov A., Mookhtiar A.K., Luu H.S., Ordureau A., Pan H., Amin P.P., Li Y., Lu Q., Yuan J. TAM kinases promote necroptosis by regulating oligomerization of MLKL. Mol. Cell. 2019;75:457–468.e4. doi: 10.1016/j.molcel.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 104.Bai L., Yu H., Wang H., Su H., Zhao J., Zhao Y. Genetic single-nucleotide polymorphisms of inflammation-related factors associated with risk of lung cancer. Med. Oncol. 2013;30:414. doi: 10.1007/s12032-012-0414-6. [DOI] [PubMed] [Google Scholar]
- 105.Watanabe S., Inoue M., Suzuki T., Kondo Y., Murayama M. Polyphyllin D induces necroptosis in neuroblastoma cells (IMR-32 and LA-N-2) in mice. Pediatr. Surg. Int. 2023;39:196. doi: 10.1007/s00383-023-05425-x. [DOI] [PubMed] [Google Scholar]
- 106.Liu X., Sun Z., Li D., Deng J., Liu J., Ma K., Qin S., Si Y., Zhang T., Feng T., Liu Y., Tan Y. Polyphyllin I inhibits invasion and epithelial-mesenchymal transition via CIP2A/PP2A/ERK signaling in prostate cancer. Int. J. Oncol. 2018 doi: 10.3892/ijo.2018.4464. [DOI] [PubMed] [Google Scholar]
- 107.Mehrpour M., Esclatine A., Beau I., Codogno P. Overview of macroautophagy regulation in mammalian cells. Cell Res. 2010;20:748–762. doi: 10.1038/cr.2010.82. [DOI] [PubMed] [Google Scholar]
- 108.Retraction J. Cell. Physiol. 2022;237:2600. doi: 10.1002/jcp.30741. 2600. [DOI] [PubMed] [Google Scholar]
- 109.Carling D. AMPK signalling in health and disease. Curr. Opin. Cell Biol. 2017;45:31–37. doi: 10.1016/j.ceb.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 110.Tanida I., Ueno T., Kominami E. In: Autophagosome and Phagosome. Deretic V., editor. Humana Press; Totowa, NJ: 2008. LC3 and autophagy; pp. 77–88. [DOI] [Google Scholar]
- 111.Gao W., Chen Z., Wang W., Stang M.T. E1-Like activating enzyme Atg7 is preferentially sequestered into p62 aggregates via its interaction with LC3-I. PLoS One. 2013;8 doi: 10.1371/journal.pone.0073229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu Y., Si Y., Xiang Y., Zhou T., Liu X., Wu M., Li W., Zhang T., Xiang K., Zhang L., Zhao H., Liu Y. Polyphyllin I activates AMPK to suppress the growth of non-small-cell lung cancer via induction of autophagy. Arch. Biochem. Biophys. 2020;687 doi: 10.1016/j.abb.2020.108285. [DOI] [PubMed] [Google Scholar]
- 113.Xie Z.-Z., Li M.-M., Deng P.-F., Wang S., Wang L., Lu X.-P., Hu L.-B., Chen Z., Jie H.-Y., Wang Y.-F., Liu X.-X., Liu Z. Paris saponin-induced autophagy promotes breast cancer cell apoptosis via the Akt/mTOR signaling pathway. Chem. Biol. Interact. 2017;264:1–9. doi: 10.1016/j.cbi.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 114.Luo Q., Jia L., Huang C., Qi Q., Jahangir A., Xia Y., Liu W., Shi R., Tang L., Chen Z. Polyphyllin I promotes autophagic cell death and apoptosis of colon cancer cells via the ROS-inhibited AKT/mTOR pathway. Indian J. Manag. Sci. 2022;23:9368. doi: 10.3390/ijms23169368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li Y., Liu H., Liu X., Xiao B., Zhang M., Luo Y., Li M., Yang J. Gracillin shows potential efficacy against non-small cell lung cancer through inhibiting the mTOR pathway. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.851300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yuan Y.-L., Jiang N., Li Z.-Y., Song Z.-Z., Yang Z.-H., Xue W.-H., Zhang X.-J., Du Y. Polyphyllin VI induces apoptosis and autophagy in human osteosarcoma cells by modulation of ROS/JNK activation. DDDT. 2019;13:3091–3103. doi: 10.2147/DDDT.S194961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cheng A., Tse K.-H., Chow H.-M., Gan Y., Song X., Ma F., Qian Y.X.Y., She W., Herrup K. ATM loss disrupts the autophagy-lysosomal pathway. Autophagy. 2021;17:1998–2010. doi: 10.1080/15548627.2020.1805860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li X., Liu Y., Liao S., Lin C., Moro A., Liu J., Feng W., Wang K., Wang C. Polyphyllin VII induces apoptosis and autophagy via mediating H2O2 levels and the JNK pathway in human osteosarcoma U2OS cells. Oncol. Rep. 2020;45:180–190. doi: 10.3892/or.2020.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kaushik S., Cuervo A.M. The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell Biol. 2018;19:365–381. doi: 10.1038/s41580-018-0001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dong R., Qin C., Yin Y., Han L., Xiao C., Wang K., Wei R., Xia Y., Kong L. Discovery of a potent inhibitor of chaperone‐mediated autophagy that targets the HSC70–LAMP2A interaction in non‐small cell lung cancer cells. Br. J. Pharmacol. 2023 doi: 10.1111/bph.16165. bph.16165. [DOI] [PubMed] [Google Scholar]
- 121.Yang W.S., SriRamaratnam R., Welsch M.E., Shimada K., Skouta R., Viswanathan V.S., Cheah J.H., Clemons P.A., Shamji A.F., Clish C.B., Brown L.M., Girotti A.W., Cornish V.W., Schreiber S.L., Stockwell B.R. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ding Y., Chen X., Liu C., Ge W., Wang Q., Hao X., Wang M., Chen Y., Zhang Q. Identification of a small molecule as inducer of ferroptosis and apoptosis through ubiquitination of GPX4 in triple negative breast cancer cells. J. Hematol. Oncol. 2021;14:19. doi: 10.1186/s13045-020-01016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hu C., Zu D., Xu J., Xu H., Yuan L., Chen J., Wei Q., Zhang Y., Han J., Lu T., Dong J., Qin J.-J., Xu Z., Cheng X. Polyphyllin B suppresses gastric tumor growth by modulating iron metabolism and inducing ferroptosis. Int. J. Biol. Sci. 2023;19:1063–1079. doi: 10.7150/ijbs.80324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kerins M.J., Ooi A. The roles of NRF2 in modulating cellular iron homeostasis. Antioxidants Redox Signal. 2018;29:1756–1773. doi: 10.1089/ars.2017.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tian Y., Lu J., Hao X., Li H., Zhang G., Liu X., Li X., Zhao C., Kuang W., Chen D., Zhu M. FTH1 inhibits ferroptosis through ferritinophagy in the 6-OHDA model of Parkinson's disease. Neurotherapeutics. 2020;17:1796–1812. doi: 10.1007/s13311-020-00929-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang R., Gao W., Wang Z., Jian H., Peng L., Yu X., Xue P., Peng W., Li K., Zeng P. Polyphyllin I induced ferroptosis to suppress the progression of hepatocellular carcinoma through activation of the mitochondrial dysfunction via Nrf2/HO-1/GPX4 axis. Phytomedicine. 2024;122 doi: 10.1016/j.phymed.2023.155135. [DOI] [PubMed] [Google Scholar]
- 127.Zheng F., Wang Y., Zhang Q., Chen Q., Liang C.-L., Liu H., Qiu F., Chen Y., Huang H., Lu W., Dai Z. Polyphyllin I suppresses the gastric cancer growth by promoting cancer cell ferroptosis. Front. Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1145407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zheng F., Bi J.-C., Wei Y.-Y., Wang Y., Zhang Q., Liang C.-L., Wu J., Dai Z. MiR-124-3p mediates gastric cancer cell ferroptosis induced by an anti-cancer drug polyphyllin I. Front. Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1285799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yu Y., Yan Y., Niu F., Wang Y., Chen X., Su G., Liu Y., Zhao X., Qian L., Liu P., Xiong Y. Ferroptosis: a cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Dis. 2021;7:193. doi: 10.1038/s41420-021-00579-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ren J.-X., Li C., Yan X.-L., Qu Y., Yang Y., Guo Z.-N. Crosstalk between oxidative stress and ferroptosis/oxytosis in ischemic stroke: possible targets and molecular mechanisms. Oxid. Med. Cell. Longev. 2021;2021:1–13. doi: 10.1155/2021/6643382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee S., Hwang N., Seok B.G., Lee S., Lee S.-J., Chung S.W. Autophagy mediates an amplification loop during ferroptosis. Cell Death Dis. 2023;14:464. doi: 10.1038/s41419-023-05978-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu J., Kuang F., Kroemer G., Klionsky D.J., Kang R., Tang D. Autophagy-dependent ferroptosis: machinery and regulation. Cell Chem. Biol. 2020;27:420–435. doi: 10.1016/j.chembiol.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huang H., Lee M.-H., Liu K., Dong Z., Ryoo Z., Kim M.O. PBK/TOPK: an effective drug target with diverse therapeutic potential. Cancers. 2021;13:2232. doi: 10.3390/cancers13092232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xiang Y., Wan F., Ren Y., Yang D., Xiang K., Zhu B., Ruan X., Li S., Zhang L., Liu X., Si Y., Liu Y. Polyphyllin VII induces autophagy‐dependent ferroptosis in human gastric cancer through targeting T‐lymphokine‐activated killer cell‐originated protein kinase. Phytother Res. 2023;37:5803–5820. doi: 10.1002/ptr.7986. [DOI] [PubMed] [Google Scholar]
- 135.Mussbacher M., Salzmann M., Brostjan C., Hoesel B., Schoergenhofer C., Datler H., Hohensinner P., Basílio J., Petzelbauer P., Assinger A., Schmid J.A. Cell type-specific roles of NF-κB linking inflammation and thrombosis. Front. Immunol. 2019;10:85. doi: 10.3389/fimmu.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gopinath V.K., Musa M., Samsudin A.R., Sosroseno W. Role of interleukin-1β and tumour necrosis factor-α on hydroxyapatite-induced phagocytosis by murine macrophages (RAW264.7 cells) Br. J. Biomed. Sci. 2006;63:176–178. doi: 10.1080/09674845.2006.11978094. [DOI] [PubMed] [Google Scholar]
- 137.Moncada S. Nitric oxide: discovery and impact on clinical medicine. J. R. Soc. Med. 1999;92:164–169. doi: 10.1177/014107689909200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fearon U., Canavan M., Biniecka M., Veale D.J. Hypoxia, mitochondrial dysfunction and synovial invasiveness in rheumatoid arthritis. Nat. Rev. Rheumatol. 2016;12:385–397. doi: 10.1038/nrrheum.2016.69. [DOI] [PubMed] [Google Scholar]
- 139.Huang Q., Ma Y., Adebayo A., Pope R.M. Increased macrophage activation mediated through toll‐like receptors in rheumatoid arthritis. Arthritis Rheum. 2007;56:2192–2201. doi: 10.1002/art.22707. [DOI] [PubMed] [Google Scholar]
- 140.Min S.-Y., Yan M., Du Y., Wu T., Khobahy E., Kwon S.-R., Taneja V., Bashmakov A., Nukala S., Ye Y., Orme J., Sajitharan D., Kim H.-Y., Mohan C. Intra-articular nuclear factor-κB blockade ameliorates collagen-induced arthritis in mice by eliciting regulatory T cells and macrophages. Clin. Exp. Immunol. 2013;172:217–227. doi: 10.1111/cei.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang Q., Zhou X., Zhao Y., Xiao J., Lu Y., Shi Q., Wang Y., Wang H., Liang Q. Polyphyllin I ameliorates collagen-induced arthritis by suppressing the inflammation response in macrophages through the NF-κB pathway. Front. Immunol. 2018;9:2091. doi: 10.3389/fimmu.2018.02091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.W. Han, G. Hou, L. Liu, Polyphyllin I (PPI) increased the sensitivity of hepatocellular carcinoma HepG2 cells to chemotherapy., Int. J. Clin. Exp. Med. 8 (n.d.) 20664-20669. . [PMC free article] [PubMed]
- 143.Zhang C., Li C., Jia X., Wang K., Tu Y., Wang R., Liu K., Lu T., He C. In vitro and in vivo anti-inflammatory effects of polyphyllin VII through downregulating MAPK and NF-κB pathways. Molecules. 2019;24:875. doi: 10.3390/molecules24050875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kaminska J., Kowalska M., Kotowicz B., Fuksiewicz M., Glogowski M., Wojcik E., Chechlinska M., Steffen J. Pretreatment serum levels of cytokines and cytokine receptors in patients with non-small cell lung cancer, and correlations with clinicopathological features and prognosis. Oncology. 2006;70:115–125. doi: 10.1159/000093002. [DOI] [PubMed] [Google Scholar]
- 145.Shishodia S., Koul D., Aggarwal B.B. Cyclooxygenase (COX)-2 inhibitor celecoxib abrogates TNF-induced NF-κB activation through inhibition of activation of IκBα kinase and Akt in human non-small cell lung carcinoma: correlation with suppression of COX-2 synthesis. J. Immunol. 2004;173:2011–2022. doi: 10.4049/jimmunol.173.3.2011. [DOI] [PubMed] [Google Scholar]
- 146.Jin X., Demere Z., Nair K., Ali A., Ferraro G.B., Natoli T., Deik A., Petronio L., Tang A.A., Zhu C., Wang L., Rosenberg D., Mangena V., Roth J., Chung K., Jain R.K., Clish C.B., Vander Heiden M.G., Golub T.R. A metastasis map of human cancer cell lines. Nature. 2020;588:331–336. doi: 10.1038/s41586-020-2969-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dart A.E., Gordon-Weeks P.R. In: Drebrin. Shirao T., Sekino Y., editors. Springer Japan; Tokyo: 2017. The role of drebrin in cancer cell invasion; pp. 375–389. [DOI] [PubMed] [Google Scholar]
- 148.Sabouni E., Nejad M.M., Mojtabavi S., Khoshdooz S., Mojtabavi M., Nadafzadeh N., Nikpanjeh N., Mirzaei S., Hashemi M., Aref A.R., Khorrami R., Nabavi N., Ertas Y.N., Salimimoghadam S., Zandieh M.A., Rahmanian P., Taheriazam A., Hushmandi K. Unraveling the function of epithelial-mesenchymal transition (EMT) in colorectal cancer: metastasis, therapy response, and revisiting molecular pathways. Biomed. Pharmacother. 2023;160 doi: 10.1016/j.biopha.2023.114395. [DOI] [PubMed] [Google Scholar]
- 149.Chambers A.F., Matrisian L.M. Changing views of the role of matrix metalloproteinases in metastasis. JNCI Journal of the National Cancer Institute. 1997;89:1260–1270. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- 150.Kleiner D.E., Stetler-Stevenson W.G. Matrix metalloproteinases and metastasis. Cancer Chemother. Pharmacol. 1999;43:S42–S51. doi: 10.1007/s002800051097. [DOI] [PubMed] [Google Scholar]
- 151.Hidalgo M., Eckhardt S.G. Development of matrix metalloproteinase inhibitors in cancer therapy. JNCI Journal of the National Cancer Institute. 2001;93:178–193. doi: 10.1093/jnci/93.3.178. [DOI] [PubMed] [Google Scholar]
- 152.He H., Zheng L., Sun Y.-P., Zhang G.-W., Yue Z.-G. Steroidal saponins from Paris polyphylla suppress adhesion, migration and invasion of human lung cancer A549 cells via down-regulating MMP-2 and MMP-9. Asian Pac. J. Cancer Prev. APJCP. 2015;15:10911–10916. doi: 10.7314/APJCP.2014.15.24.10911. [DOI] [PubMed] [Google Scholar]
- 153.Pang D., Yang C., Li C., Zou Y., Feng B., Li L., Liu W., Luo Q., Chen Z., Huang C. Polyphyllin II inhibits liver cancer cell proliferation, migration and invasion through downregulated cofilin activity and the AKT/NF-κB pathway. Biology Open. 2020;9:bio046854. doi: 10.1242/bio.046854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu Y., Cao Y., Kai H., Han Y., Huang M., Gao L., Qiao H. Polyphyllin E inhibits proliferation, migration and invasion of ovarian cancer cells by down-regulating the AKT/NF-κB pathway. Biol. Pharmaceut. Bull. 2022;45:561–568. doi: 10.1248/bpb.b21-00691. [DOI] [PubMed] [Google Scholar]
- 155.Thiery J.P., Sleeman J.P. Complex networks orchestrate epithelial–mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 156.Kudo-Saito C., Shirako H., Takeuchi T., Kawakami Y. Cancer metastasis is accelerated through immunosuppression during snail-induced EMT of cancer cells. Cancer Cell. 2009;15:195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 157.Niu W., Xu L., Li J., Zhai Y., Sun Z., Shi W., Jiang Y., Ma C., Lin H., Guo Y., Liu Z. Polyphyllin II inhibits human bladder cancer migration and invasion by regulating EMT-associated factors and MMPs. Oncol. Lett. 2020;20:2928–2936. doi: 10.3892/ol.2020.11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Normanno N., De Luca A., Bianco C., Strizzi L., Mancino M., Maiello M.R., Carotenuto A., De Feo G., Caponigro F., Salomon D.S. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 159.Wee P., Wang Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers. 2017;9:52. doi: 10.3390/cancers9050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cai T., Nishida K., Hirano T., Khavari P.A. Gab1 and SHP-2 promote Ras/MAPK regulation of epidermal growth and differentiation. J. Cell Biol. 2002;159:103–112. doi: 10.1083/jcb.200205017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hof P., Pluskey S., Dhe-Paganon S., Eck M.J., Shoelson S.E. Crystal structure of the tyrosine phosphatase SHP-2. Cell. 1998;92:441–450. doi: 10.1016/S0092-8674(00)80938-1. [DOI] [PubMed] [Google Scholar]
- 162.Shi Z.-Q., Yu D.-H., Park M., Marshall M., Feng G.-S. Molecular mechanism for the shp-2 tyrosine phosphatase function in promoting growth factor stimulation of Erk activity. Mol. Cell Biol. 2000;20:1526–1536. doi: 10.1128/MCB.20.5.1526-1536.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kwon S.J., Ahn D., Yang H.-M., Kang H.J., Chung S.J. Polyphyllin D shows anticancer effect through a selective inhibition of Src homology region 2-containing protein tyrosine phosphatase-2 (SHP2) Molecules. 2021;26:848. doi: 10.3390/molecules26040848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lin D., Shen L., Luo M., Zhang K., Li J., Yang Q., Zhu F., Zhou D., Zheng S., Chen Y., Zhou J. Circulating tumor cells: biology and clinical significance. Signal Transduct. Targeted Ther. 2021;6:404. doi: 10.1038/s41392-021-00817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Han H., Sung J.Y., Kim S.-H., Yun U.-J., Kim H., Jang E.-J., Yoo H.-E., Hong E.K., Goh S.-H., Moon A., Lee J.-S., Ye S.-K., Shim J., Kim Y.-N. Fibronectin regulates anoikis resistance via cell aggregate formation. Cancer Lett. 2021;508:59–72. doi: 10.1016/j.canlet.2021.03.011. [DOI] [PubMed] [Google Scholar]
- 166.Que Z., Luo B., Yu P., Qi D., Shangguan W., Wang P., Liu J., Li Y., Li H., Ke R., Wu E., Tian J. Polyphyllin VII induces CTC anoikis to inhibit lung cancer metastasis through EGFR pathway regulation. Int. J. Biol. Sci. 2023;19:5204–5217. doi: 10.7150/ijbs.83682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cao Z., Zhang R., Li J., Huang H., Zhang D., Zhang J., Gao J., Chen J., Huang C. X-Linked inhibitor of apoptosis protein (XIAP) regulation of cyclin D1 protein expression and cancer cell anchorage-independent growth via its E3 ligase-mediated protein phosphatase 2A/c-Jun Axis. J. Biol. Chem. 2013;288:20238–20247. doi: 10.1074/jbc.M112.448365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gao N., Liu J., Liu D., Hao Y., Yan L., Ma Y., Zhuang H., Hu Z., Gao J., Yang Z., Shi H., Lin B. c-Jun transcriptionally regulates alpha 1, 2-fucosyltransferase 1 (FUT1) in ovarian cancer. Biochimie. 2014;107:286–292. doi: 10.1016/j.biochi.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 169.Gu L., Feng J., Xu H., Luo M., Su D. Polyphyllin I inhibits proliferation and metastasis of ovarian cancer cell line HO-8910PM in vitro. J. Tradit. Chin. Med. 2013;33:325–333. doi: 10.1016/S0254-6272(13)60174-0. [DOI] [PubMed] [Google Scholar]
- 170.Wang W., Liu Y., You L., Sun M., Qu C., Dong X., Yin X., Ni J. Inhibitory effects of Paris saponin I, II, Ⅵ and Ⅶ on HUVEC cells through regulation of VEGFR2, PI3K/AKT/mTOR, Src/eNOS, PLCγ/ERK/MERK, and JAK2-STAT3 pathways. Biomed. Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110750. [DOI] [PubMed] [Google Scholar]
- 171.Du H., Wu H., Kang Q., Liao M., Qin M., Chen N., Huang H., Huang D., Wang P., Tong G. Polyphyllin I attenuates the invasion and metastasis via downregulating GRP78 in drug-resistant hepatocellular carcinoma cells. Aging. 2023;15:12251–12263. doi: 10.18632/aging.205176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yang Q., Guo J., Zheng J., Chen Y., Zou B., Li R., Ding Z., Wang Y., Li L., Chen Z., Mo L., Liang Q., Chen F., Li X. Polyphyllin VII protects from breast cancer-induced osteolysis by suppressing osteoclastogenesis via c-Fos/NFATc1 signaling. Int. Immunopharm. 2023;120 doi: 10.1016/j.intimp.2023.110316. [DOI] [PubMed] [Google Scholar]
- 173.Yang L., Zhu T., Ye H., Shen Y., Li Z., Chen L., Wang C., Chen X., Zhao H., Xiang Y., Xiao Z., Zhao C., Li J., Hu W. Gracillin shows potent efficacy against colorectal cancer through inhibiting the STAT3 pathway. J. Cell Mol. Med. 2021;25:801–812. doi: 10.1111/jcmm.16134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Maniotis A.J., Folberg R., Hess A., Seftor E.A., Gardner L.M.G., Pe’er J., Trent J.M., Meltzer P.S., Hendrix M.J.C. Vascular Channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am. J. Pathol. 1999;155:739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Czekierdowski A., Czekierdowska S., Stachowicz N., Łoziński T., Gurynowicz G. Mig-7 expression and vasculogenic mimicry in malignant ovarian tumors. Ginekol. Pol. 2017;88:552–561. doi: 10.5603/GP.a2017.0100. [DOI] [PubMed] [Google Scholar]
- 176.Robertson G.P. Mig-7 linked to vasculogenic mimicry. Am. J. Pathol. 2007;170:1454–1456. doi: 10.2353/ajpath.2007.070127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yao N., Zhou J., Jiang Y., Jin Q., Zhu H., Zhang J., Li Z. Rhizoma Paridis saponins suppresses vasculogenic mimicry formation and metastasis in osteosarcoma through regulating miR-520d-3p/MIG-7 axis. J. Pharmacol. Sci. 2022;150:180–190. doi: 10.1016/j.jphs.2022.08.005. [DOI] [PubMed] [Google Scholar]
- 178.Fan Y.-L., Zheng M., Tang Y.-L., Liang X.-H. A new perspective of vasculogenic mimicry: EMT and cancer stem cells. Oncol. Lett. 2013;6:1174–1180. doi: 10.3892/ol.2013.1555. (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xiao T., Zhong W., Zhao J., Qian B., Liu H., Chen S., Qiao K., Lei Y., Zong S., Wang H., Liang Y., Zhang H., Meng J., Zhou H., Sun T., Liu Y., Yang C. Polyphyllin I suppresses the formation of vasculogenic mimicry via Twist1/VE-cadherin pathway. Cell Death Dis. 2018;9:906. doi: 10.1038/s41419-018-0902-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xiao X., Yang M., Xiao J., Zou J., Huang Q., Yang K., Zhang B., Yang F., Liu S., Wang H., Bai P. Paris Saponin II suppresses the growth of human ovarian cancer xenografts via modulating VEGF-mediated angiogenesis and tumor cell migration. Cancer Chemother. Pharmacol. 2014;73:807–818. doi: 10.1007/s00280-014-2408-x. [DOI] [PubMed] [Google Scholar]
- 181.Sun C.-L., Ni W., Yan H., Liu Z.-H., Yang L., Si Y.-A., Hua Y., Chen C.-X., He L., Zhao J.-H., Liu H.-Y. Steroidal saponins with induced platelet aggregation activity from the aerial parts of Paris verticillata. Steroids. 2014;92:90–95. doi: 10.1016/j.steroids.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 182.Wen F., Chen T., Yin H., Lin J., Zhang H. In vitro effects on thrombin of Paris saponins and in vivo hemostatic activity evaluation of Paris fargesii var. brevipetala. Molecules. 2019;24:1420. doi: 10.3390/molecules24071420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tang X., Yang J., Li J. Accelerative effect of leflunomide on recovery from hepatic fibrosis involves TRAIL-mediated hepatic stellate cell apoptosis. Life Sci. 2009;84:552–557. doi: 10.1016/j.lfs.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 184.Bataller R., Brenner D.A. Liver fibrosis. J. Clin. Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Man S., Fan W., Gao W., Li Y., Wang Y., Liu Z., Li H. Anti-fibrosis and anti-cirrhosis effects of Rhizoma paridis saponins on diethylnitrosamine induced rats. J. Ethnopharmacol. 2014;151:407–412. doi: 10.1016/j.jep.2013.10.051. [DOI] [PubMed] [Google Scholar]
- 186.Hong Y., Han Y.-Q., Wang Y.-Z., Gao J.-R., Li Y.-X., Liu Q., Xia L.-Z. Paridis Rhizoma Sapoinins attenuates liver fibrosis in rats by regulating the expression of RASAL1/ERK1/2 signal pathway. J. Ethnopharmacol. 2016;192:114–122. doi: 10.1016/j.jep.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 187.Zampieri S., Filocamo M., Pianta A., Lualdi S., Gort L., Coll M.J., Sinnott R., Geberhiwot T., Bembi B., Dardis A. SMPD1 mutation update: database and comprehensive analysis of published and novel variants: human mutation. Hum. Mutat. 2016;37:139–147. doi: 10.1002/humu.22923. [DOI] [PubMed] [Google Scholar]
- 188.Wang Y., Chen Y.-Y., Gao G.-B., Zheng Y.-H., Yu N.-N., Ouyang L., Gao X., Li N., Wen S.-Y., Huang S., Zhao Q., Liu L., Cao M., Zhang S., Zhang J., He Q.-Y. Polyphyllin D punctures hypertrophic lysosomes to reverse drug resistance of hepatocellular carcinoma by targeting acid sphingomyelinase. Mol. Ther. 2023;31:2169–2187. doi: 10.1016/j.ymthe.2023.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chen Q., Sun L., Chen Z.J. Regulation and function of the cGAS–STING pathway of cytosolic DNA sensing. Nat. Immunol. 2016;17:1142–1149. doi: 10.1038/ni.3558. [DOI] [PubMed] [Google Scholar]
- 190.Mantovani A., Marchesi F., Malesci A., Laghi L., Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yu J., Deng H., Xu Z. Targeting macrophage priming by polyphyllin VII triggers anti-tumor immunity via STING-governed cytotoxic T-cell infiltration in lung cancer. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-77800-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wang W., Liu Y., Sun M., Sai N., You L., Dong X., Yin X., Ni J. Hepatocellular toxicity of Paris saponins I, II, VI and VII on two kinds of hepatocytes-HL-7702 and HepaRG cells, and the underlying mechanisms. Cells. 2019;8:690. doi: 10.3390/cells8070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Li Z., Fan Q., Chen M., Dong Y., Li F., Wang M., Gu Y., Guo S., Ye X., Wu J., Dai S., Lin R., Zhao C. The interaction between polyphyllin I and SQLE protein induces hepatotoxicity through SREBP-2/HMGCR/SQLE/LSS pathway. Journal of Pharmaceutical Analysis. 2023;13:39–54. doi: 10.1016/j.jpha.2022.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Liu Z., Gao W., Man S., Wang J., Li N., Yin S., Wu S., Liu C. Pharmacological evaluation of sedative–hypnotic activity and gastro-intestinal toxicity of Rhizoma Paridis saponins. J. Ethnopharmacol. 2012;144:67–72. doi: 10.1016/j.jep.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 195.Liu J., Liu Y., Li H., Wei C., Mao A., Liu W., Pan G. Polyphyllin D induces apoptosis and protective autophagy in breast cancer cells through JNK1-Bcl-2 pathway. J. Ethnopharmacol. 2022;282 doi: 10.1016/j.jep.2021.114591. [DOI] [PubMed] [Google Scholar]
- 196.Man S., Li Y., Fan W., Gao W., Liu Z., Li N., Zhang Y., Liu C. Curcuma increasing antitumor effect of Rhizoma paridis saponins through absorptive enhancement of paridis saponins. Int. J. Pharm. 2013;454:296–301. doi: 10.1016/j.ijpharm.2013.06.079. [DOI] [PubMed] [Google Scholar]
- 197.Liu Z., Gao W., Man S., Zhang Y., Li H., Wu S., Zhang J., Liu C. Synergistic effects of Rhizoma Paridis and Rhizoma Curcuma longa on different animal tumor models. Environ. Toxicol. Pharmacol. 2014;38:31–40. doi: 10.1016/j.etap.2014.04.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data availability is not applicable to this article as no new data were created or analyzed in this study.