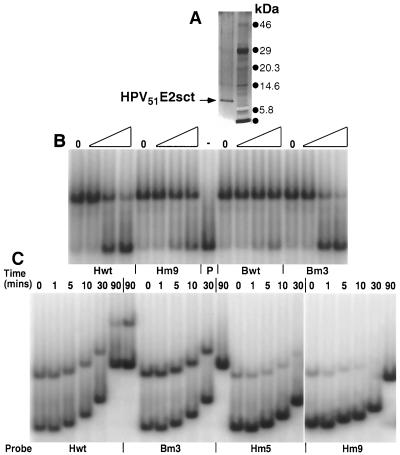
FIG. 4.
Determination of the Krel and T1/2 for the HPV51E2sct protein with various DNA binding sites. (A) Silver-stained 4 to 12% gradient SDS-PAGE of the highly purified HPV51E2sct protein preparation (lane 1). Proteins in lane 2 are prestained molecular size markers. (B) EMSAs to determine the relative affinity of the E2 protein for various DNA probes. Reaction mixtures were made with the HPV51E2sct protein and equal amounts of radiolabeled Bm#3 probe DNA with no competitor DNAs (lanes 0) or with sequential dilutions corresponding to a 2-, 100-, and 500-fold excess of the PCR-amplified unlabeled competitor DNA listed below the EMSAs. Binding was for 30 min before electrophoresis. Gels were dried, exposed to PhosporImager screens, and analyzed using ImageQuant software. (C) EMSAs to determine the rate of E2 protein-DNA dissociation. The HPV51E2sct protein was reacted for 30 min with the probe DNAs identified below each section of the EMSA. Then, a 1,000-fold excess of unlabeled competitor DNA, Bm#3, was added to each reaction mixture. Lanes 0 were loaded immediately, while others were allowed to incubate for 1, 5, 10, 30, or 90 min before electrophoresis. Gels were dried, exposed to PhosphorImager screens, and analyzed using ImageQuant software.