Fig. 3.
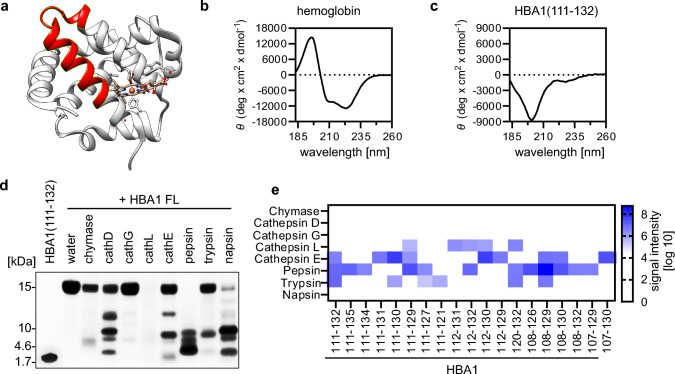
Biochemical analyses and origin of HBA1(111–132). a Visualization of full-length hemoglobin with amino acid sequence 111–132 highlighted in red. Structure derived from PDB: 6BB5. b–c Circular dichroism spectrum of full-length recombinant HBA1 (b) and HBA1(111–132) (c) in 10 mM PBS pH = 7.4 at 25 °C. d Exemplary SDS-PAGE of digested full-length hemoglobin with indicated proteases. Stained using Coomassie Brilliant Blue. HBA1 FL, Hemoglobin A full-length. e Identification of HBA1 fragments from mass spectrometry analysis of gel section at expected height of HBA1(111–132) in (d). Color code represents the signal intensity as analyzed by label-free mass spectrometry. Cathepsin (cath)