Abstract
Background and aim:
To investigate the prognostic implication of body mass index (BMI) on clinical outcomes after acute ischemic and hemorrhagic stroke.
Methods:
The subjects of the study included adult patients with available baseline body weight and height data who had suffered an acute stroke and were registered in the Japan Stroke Data Bank—a hospital-based, multicenter stroke registration database—between January 2006 and December 2020. The outcome measures included unfavorable outcomes defined as a modified Rankin Scale (mRS) score of 5–6 and favorable outcomes (mRS 0–2) at discharge, and in-hospital mortality. Mixed effects logistic regression analysis was conducted to determine the relationship between BMI categories (underweight, normal weight, overweight, class I obesity, class II obesity; <18.5, 18.5–23.0, 23.0–25.0, 25–30, ⩾30 kg/m2) and the outcomes, after adjustment for covariates.
Results:
A total of 56,230 patients were assigned to one of the following groups: ischemic stroke (IS, n = 43,668), intracerebral hemorrhage (ICH, n = 9741), and subarachnoid hemorrhage (SAH, n = 2821). In the IS group, being underweight was associated with an increased likelihood of unfavorable outcomes (odds ratio, 1.47 (95% confidence interval (CI):1.31−1.65)) and in-hospital mortality (1.55 (1.31−1.83)) compared to outcomes in those with normal weight. Being overweight was associated with an increased likelihood of favorable outcomes (1.09 (1.01−1.18)). Similar associations were observed between underweight and these outcomes in specific IS subtypes (cardioembolic stroke, large artery stroke, and small-vessel occlusion). Patients with a BMI ⩾30.0 kg/m2 was associated with an increased likelihood of unfavorable outcomes (1.44 (1.01−2.17)) and in-hospital mortality (2.42 (1.26−4.65)) in large artery stroke. In patients with ICH, but not those with SAH, being underweight was associated with an increased likelihood of unfavorable outcomes (1.41 (1.01−1.99)).
Conclusions:
BMI substantially impacts functional outcomes following IS and ICH. Lower BMI consistently affected post-stroke disability and mortality, while higher BMI values similarly affected these outcomes after large artery stroke.
Keywords: BMI, stroke subtypes, ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage, stroke
Introduction
Stroke is the second leading cause of death and the third leading cause of disability worldwide.1,2 Given the enormous burden of stroke-related disability and mortality, identifying modifiable risk factors associated with stroke outcomes is becoming increasingly imperative. Body mass index (BMI) is defined as the body weight in kilograms divided by the height in meters squared and is an estimate of total adiposity and muscle mass. Having a high BMI is a well-established risk factor for the development of cardiovascular events, including stroke, and is an independent predictor of mortality in the general population. 3 A recent systematic analysis revealed that a high BMI was one of the leading risk factors contributing to the increase in stroke-related disability-adjusted life years over the past two decades. 2 However, the prognostic impact of BMI on clinical outcomes after stroke remains inconclusive. Previous studies have shown that being overweight or obese may be associated with a favorable prognosis; this is known as the “obesity paradox” and is observed in patients afflicted by a variety of vascular diseases (e.g. coronary artery disease and heart failure) and in those with nonvascular diseases. 4 In terms of stroke, several studies have demonstrated a lower risk of stroke recurrence and all-cause mortality in overweight or obese patients compared with the outcomes in patients with a normal weight, and those studies have mostly focused on the risk of mortality. 5 A recent meta-analysis of patients with acute ischemic stroke (IS) revealed that compared with the outcomes in those with normal body weight, being underweight was associated with a higher risk of disability and mortality at 3 months, whereas obesity was not associated with a better prognosis. 6 In particular, studies examining the relationship between BMI and disability after hemorrhagic stroke are scarce, and those that have been conducted have reported conflicting results. 6 Although IS is classified into specific subtypes that have inherently heterogeneous pathogeneses, no studies have investigated the association between BMI and the etiologic subtypes of IS; therefore, the prognostic impact of BMI on functional outcomes in those suffering from IS of each etiology remains uncertain.
Accordingly, we aimed to quantify the associations between BMI and post-stroke outcomes, expressed in terms of functional outcomes after acute stroke, including ischemic and hemorrhagic stroke (intracerebral hemorrhage (ICH) and subarachnoid hemorrhage (SAH)).
Methods
Data source
Detailed information on the Japan Stroke Data Bank (JSDB) has been described in previous studies.7–9 The JSDB is an ongoing hospital-based, multicenter, prospective registry of patients treated for acute stroke or transient ischemic attack in Japan. The JSDB prospectively collects information related to the clinical diagnosis of acute stroke based on the professional evaluations of stroke specialists from 130 high-volume stroke centers and academic teaching hospitals. Data on hospitalization attributed to acute stroke are recorded by the study physicians or clinical report coordinators in each institute using a standardized database form online. The study protocol was approved by the institutional ethics review board at the National Cerebral and Cardiovascular Center (M27-090-15). Due to the anonymous nature of the data, the requirement for individual consent for entry of information into the database was waived by the institution. Instead, an opt-out consent method was employed to notify or disclose the research information.
Patients
For the analyses, adult patients (⩾18 years) diagnosed with acute ischemic or hemorrhagic stroke who had available body weight and height data at admission were eligible for inclusion. The database included clinical information on demographic variables, stroke subtypes (IS including IS subtypes, ICH, and SAH), according to the International Classification of Diseases, Tenth Revision (codes 160, 161, 163), neurological impairment (National Institutes of Health Stroke Scale (NIHSS) for patients with IS or ICH and World Federation of Neurological Surgeons (WFNS) grading for those with SAH), medical history, risk factors, medications, and functional outcomes at discharge, based on modified Rankin Scale (mRS) scores. IS was classified into the following five subtypes according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria: large artery stroke, cardioembolic stroke, small-vessel occlusion, other determined etiology stroke, or undetermined etiology stroke. 10 Height and weight upon admission were measured by nursing staff while lying flat in bed or self-reported by patients or legal proxies. Patients were grouped into five BMI categories. As recommended for Asian populations by the World Health Organization definitions and the American Heart Association, the BMI categories were slightly modified,11,12 as follows: (1) underweight: BMI <18.5; (2) normal weight:18.5 ⩽ BMI < 23; (3) overweight: 23.0 ⩽ BMI < 25; (4) class I obesity: 25.0 ⩽ BMI < 30; and (5) class II obesity: BMI ⩾30.0 kg/m2.
We evaluated the association of BMI with the following outcomes by stroke subtypes. The primary outcome measure was an unfavorable functional outcome at discharge, defined as mRS score of 5–6. The secondary outcomes were the following: (1) a favorable outcome, defined as mRS score of 0–2; (2) distribution of mRS score; and (3) in-hospital all-cause mortality.
Statistical analysis
Descriptive statistics were used to summarize the baseline characteristics of the study population following stratification based on BMI. Continuous data were presented as the mean (SD) or median (interquartile range), whereas categorical data were presented as n (%). Kruskal–Wallis, chi-square test, and Cochran–Armitage trend tests were used to evaluate the significance of differences between BMI categories for continuous and categorical variables as appropriate. Mixed effects binary logistic regression analyses using the institutions as random intercepts were performed to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) to evaluate the associations between BMI and individual outcomes. Mixed effects ordinal logistic regression analysis was employed to compare the distribution of mRS score. Risk estimates were adjusted for covariates on the basis of clinical relevance for functional outcome after stroke and availability in this study: age, sex, hypertension, diabetes, dyslipidemia, atrial fibrillation, history of stroke or cancer, premorbid mRS score, initial NIHSS (for those with IS and ICH) or WFNS grade (for those with SAH), systolic blood pressure, and acute reperfusion therapy (intravenous thrombolysis and/or endovascular treatment for those with IS) or the ICH location and hematoma volume for those with ICH, using stratified groups based on stroke types. The same covariates were adjusted across all IS subtypes. We tested the interaction effects between BMI and key characteristics (age (<60, 60–80, or ⩾80 years), sex, hypertension, diabetes, dyslipidemia, and NIHSS (0–5, 5–10, 10–20, or >20)),13,14 and reperfusion therapies for IS by including the respective interaction terms in the final model. Restricted cubic splines were used to allow for non-linear relationships between BMI as continuous variables and outcomes to facilitate calculations of the ORs at each BMI increment. The knots for the splines were set at 18.5, 23, 25, and 30 kg/m2, which correspond to the cut-off points for underweight, normal weight, overweight, and obesity categories, respectively.11,12 A BMI of 23 kg/m2 was used as the reference. The analyses were based on data from all patients who had complete information; therefore, missing values were not imputed. We acknowledged the over-inflation of type I error probability resulting from multiple comparisons. As this work was considered to be hypothesis generating, all p values were two-sided, and a p value of <0.05 was considered statistically significant. The analyses were performed using STATA17 (Stata Corp).
Results
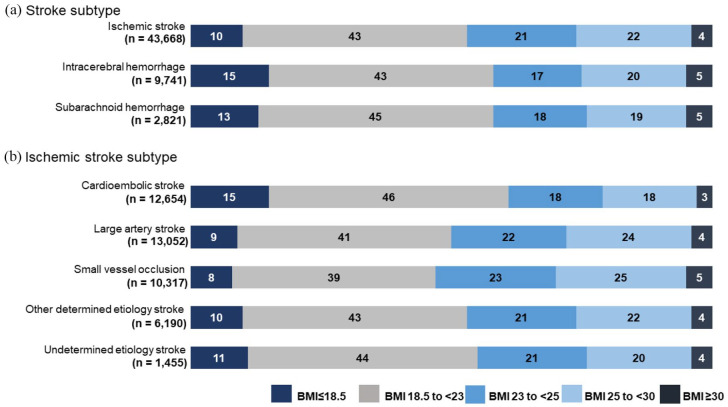
The subjects were abstracted from patients treated from January 2006 (the time at which the collection of data on body weight and height at admission was conducted) to December 2020; among these, BMI assessments were conducted in 56,230 patients (32%), whereas no BMI data existed for 119,625 patients (Figure S1). The comparison of demographic and clinical data between those with available BMI data and those without it is shown in Supplemental result and Table S1–3. Accordingly, 56,230 patients were included in this study, 43,668 of whom were diagnosed with IS (mean age: 74 ± 12 years; 26,696 (61%) males), 9741 of whom were diagnosed with ICH (mean age: 69 ± 14 years; 5654 (56%) males), and 2821 of whom were diagnosed with SAH (mean age: 63 ± 15 years; 943 (33%) males). The patient distributions according to BMI categories are shown in Figure 1.
Figure 1.
Percentages of individuals in each of BMI categories by (a) stroke type and (b) ischemic stroke subtype.
Associations between BMI and clinical outcomes in IS
The clinical characteristics according to the BMI categories among those with IS are presented in Table 1. The patients with higher BMI tended to be younger in age and current smokers, with more frequent drinking habits and a metabolic risk profile (hypertension, diabetes, dyslipidemia) that led to more frequent treatment with pharmacotherapeutic agents (i.e. antihypertensive and antidiabetic agents). However, atrial fibrillation and a history of stroke or cancer were less common in those with higher BMI. The initial NIHSS score was higher, the duration of hospitalization was longer, and the baseline systolic blood pressure and diastolic blood pressure were lower in those who were underweight than in those with obesity.
Table 1.
Comparison of clinical characteristics between BMI categories in patients with ischemic stroke.
| Underweight (BMI < 18.5) (n = 4539) | Normal weight (18.5 ⩽ BMI < 23) (n = 18,550) | Overweight (23.0 ⩽ BMI < 25) (n = 9130) | Class I obesity (25.0 ⩽ BMI < 30) (n = 9689) | Class II obesity (BMI ⩾ 30) (n = 1760) | p | |
|---|---|---|---|---|---|---|
| Age, years | 79.9 ± 11.7 | 75.6 ± 11.9 | 72.5 ± 11.5 | 70.1 ± 12.2 | 64.5 ± 14.2 | <0.001 |
| Male sex | 1902 (41) | 10,782 (58) | 6372 (69) | 6606 (68) | 1033 (59) | <0.001 |
| Premorbid mRS score | 1 (0−2) | 0 (0−1) | 0 (0−1) | 0 (0−1) | 0 (0−0) | <0.001 |
| Hypertension | 2821 (63) | 12,576 (68) | 6662 (73) | 7458 (77) | 1442 (82) | <0.001 |
| Diabetes | 801 (17) | 4525 (24) | 2750 (30) | 3349 (24) | 769 (43) | <0.001 |
| Dyslipidemia | 1086 (24) | 6478 (35) | 3866 (42) | 4564 (47) | 929 (52) | <0.001 |
| Atrial fibrillation | 1332 (30) | 4204 (23) | 1684 (18) | 1683 (17) | 282 (16) | <0.001 |
| History of stroke | 1347 (30) | 5040 (27) | 2420 (27) | 2439 (25) | 362 (20) | <0.001 |
| History of cancer | 442 (9) | 1243 (6) | 508 (5) | 482 (5) | 72 (4) | <0.001 |
| Current smoking a | 686/4174 (16) | 3518/16,929 (20) | 1956/8262 (24) | 2178/8761 (24) | 415/1594 (26) | <0.001 |
| Drinking habits b | 200/3593 (6) | 1375/14,556 (9) | 815/7032 (11) | 953/7616 (12) | 155/1394 (4) | <0.001 |
| Antihypertensive agents c | 2211/3324 (66) | 9507/13,381 (71) | 4927/6553 (75) | 5482/7003 (78) | 989/1239 (70) | <0.001 |
| Statins c | 440/3324 (13) | 2613/13,381 (19) | 1458/6553 (22) | 1712/7003 (24) | 317/1239 (25) | <0.001 |
| Antidiabetic agents c | 440/3324 (13) | 2594/13,381 (19) | 1570/6553 (24) | 1942/7003 (27) | 419/1239 (34) | <0.001 |
| Antiplatelet agents c | 1129/3324 (34) | 4906/13,381 (36) | 2518/6553 (38) | 2597/7003 (37) | 386/1239 (31) | 0.452 |
| Anticoagulants c | 704/3324 (21) | 241/13,381 (18) | 1026/6553 (15) | 1016/7003 (14) | 151/1239 (12) | <0.001 |
| Systolic BP, mmHg | 154 ± 29 | 157 ± 28 | 159 ± 28 | 161 ± 28 | 165 ± 28 | <0.001 |
| Diastolic BP, mmHg | 84 ± 18 | 86 ± 18 | 88 ± 18 | 89 ± 18 | 94 ± 20 | <0.001 |
| NIHSS | 6 (3−16) | 4 (2−10) | 3 (1−7) | 3 (1−6) | 3 (1−5) | <0.001 |
| Acute reperfusion therapy | 619 (13) | 2317 (12) | 1121 (12) | 1095 (11) | 208 (11) | 0.384 |
| mRS score | 4 (2−5) | 2 (1−4) | 2 (1−4) | 2 (1−3) | 1 (1−3) | <0.001 |
| mRS 0–2 | 1561 (34) | 9940 (54) | 5751 (63) | 6258 (65) | 1170 (67) | <0.001 |
| mRS 5–6 | 1332 (29) | 2900 (15) | 936 (10) | 919 (9) | 148 (8) | <0.001 |
| In-hospital mortality | 357 (8) | 686 (4) | 220 (2) | 219 (2) | 44 (3) | <0.001 |
| Duration of hospitalization, days | 19 (11−32) | 16 (10−28) | 15 (10−24) | 15 (10−24) | 15 (9−23) | <0.001 |
Acute reperfusion therapy includes alteplase use or/and endovascular treatment. Drinking habits was reported as current drinker and characterized based on total weekly intake as >8 drinks. Values are presented as frequencies (percentages), means ± SD, or medians (interquartile ranges).
Abbreviations: mRS, modified Rankin scale; BP, blood pressure; NIHSS, National Institutes of Health Stroke Scale.
Data on current smoking habits were available for 36,666 patients.
Data on drinking habits were available for 34,191 patients.
Data on the use of antihypertensive agents, statins, antidiabetic agents, antiplatelet agents, and anticoagulants were available for 30,818 patients.
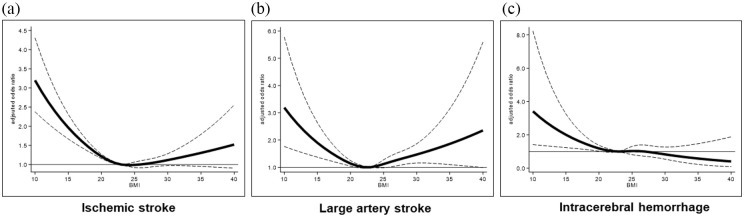
Figure S2 shows the proportions of individuals with the mRS scores for overall IS and for each IS subtype. An inverse linear trend was observed between unfavorable outcomes and BMI categories for all IS subtypes (p for trend <0.001). In the multivariable analysis, underweight patients had a higher risk of experiencing unfavorable outcomes than did those with normal weight in IS and all IS subtypes (Table 2). In addition, being overweight was inversely associated with unfavorable outcomes in IS (OR, 0.87 (95% CI, 0.78−0.98)) and small-vessel occlusion (0.59 (0.37−0.95)) (Table 2). Patients with a BMI ⩾30.0 kg/m2 were positively associated with unfavorable outcomes in large artery stroke (1.44 (1.01−2.17)) (Table 2). In the adjusted restricted cubic spline analysis, a lower BMI (<23 kg/m2) was associated with unfavorable outcomes in IS and in all IS subtypes (Figure 2(a) and (b), Figure S3). In addition, a significant U-shaped association was observed in those with large artery stroke (Figure 2(b)). The subgroup analysis revealed significant BMI–subgroup interactions for age, sex, hypertension, stroke severity, and acute reperfusion therapy (Table S4). The effect of underweight on unfavorable outcomes was significant in patients with higher NIHSS (⩾10) (1.58 (1.28−1.95)) and those receiving acute reperfusion therapy (1.46 (1.31−1.64)), while an inverse association was observed between unfavorable outcomes and obesity in older patients (age ⩾80 years) (0.83 (0.70−0.97)) (Table S4).
Table 2.
Associations between BMI categories and outcome measures in ischemic stroke subgroups.
| Underweight (BMI < 18.5) | Normal weight (18.5 ⩽ BMI < 23) | Overweight (23.0 ⩽ BMI < 25) | Class I obesity (25.0 ⩽ BMI < 30) | Class II obesity (BMI ⩾ 30) | |
|---|---|---|---|---|---|
| Unfavorable outcome (mRS 5–6) | |||||
| Overall IS | 1.47 (1.31−1.65) † | Ref | 0.87 (0.78−0.98)* | 0.92 (0.82−1.04) | 1.01 (0.78−1.29) |
| Cardioembolic stroke | 1.38 (1.17−1.62) † | Ref | 0.92 (0.78−1.09) | 0.88 (0.78−1.09) | 0.92 (0.63−1.35) |
| Large artery stroke | 1.51 (1.19−1.90) † | Ref | 0.95 (0.76−1.17) | 1.17 (0.98−1.44) | 1.44 (1.01−2.17)* |
| Small-vessel occlusion | 1.61 (1.09−2.39)* | Ref | 0.59 (0.37−0.95)* | 0.89 (0.57−1.38) | 0.89 (0.34−2.32) |
| Other determined etiology stroke | 1.42 (1.04−1.91)* | Ref | 0.76 (0.55−1.03) | 0.74 (0.53−1.04) | 0.55 (0.23−1.28) |
| Undetermined etiology stroke | 2.39 (1.24−4.59) † | Ref | 1.39 (0.69−2.80) | 1.20 (0.61−2.38) | 0.79 (0.08−7.60) |
| In-hospital mortality | |||||
| Overall IS | 1.55 (1.31−1.83) † | Ref | 0.92 (0.77−1.11) | 0.91 (0.75−1.10) | 1.23 (0.83−1.84) |
| Cardioembolic stroke | 1.45 (1.17−1.79) † | Ref | 1.05 (0.83−1.33) | 0.88 (0.68−1.13) | 0.94 (0.53−1.67) |
| Large artery stroke | 1.69 (1.13−2.53) † | Ref | 0.96 (0.63−1.44) | 1.30 (0.88−1.92) | 2.42 (1.26−4.65) † |
| Small-vessel occlusion | 2.38 (1.06−5.34) † | Ref | 0.16 (0.02−1.26) | 0.37 (0.08−1.68) | 3.77 (0.79−18.0) |
| Other determined etiology stroke | 1.59 (1.00−2.55) | Ref | 0.65 (0.33−1.21) | 0.72 (0.39−1.34) | 0.33 (0.04−2.48) |
| Undetermined etiology stroke | 1.30 (0.46−3.62) | Ref | 0.59 (0.17−2.04) | 1.15 (0.42−3.18) | 2.27 (0.22−23.2) |
Data are presented as odds ratios (95% confidence intervals) for outcomes in ischemic stroke subtypes based on BMI categories. Risk estimates were adjusted for age, sex, hypertension, diabetes, dyslipidemia, atrial fibrillation, history of stroke, history of cancer, premorbid mRS score, NIHSS, systolic blood pressure, and acute reperfusion therapy.
Abbreviations: mRS, modified Rankin scale; IS, ischemic stroke; Ref, reference.
p < 0.05, †p < 0.001.
Figure 2.
Associations between BMI and unfavorable outcomes (mRS 5–6) by stroke types. Adjusted odds ratios (ORs) for unfavorable outcomes ((a) overall ischemic stroke; (b) large artery stroke; (c) intracerebral hemorrhage) according to BMI, based on restricted cubic splines. Risk estimates were adjusted for age, sex, hypertension, diabetes, dyslipidemia, atrial fibrillation, history of stroke, history of cancer, premorbid mRS score, NIHSS, systolic blood pressure, and acute reperfusion therapy (in those with ischemic stroke) or the location and hematoma volume (in those with intracerebral hemorrhage). Solid lines represent the OR and dashed lines represent the 95% confidence interval. A BMI of 23 was used as the reference.
Similarly, the inverse association between being underweight and favorable outcomes (mRS 0–2) was observed. Being underweight was also associated with outcomes across mRS shift, and in-hospital mortality in IS subtypes, except for stroke of other determined and undetermined etiologies (Table 2, Table S5). Patients with a BMI ⩾30.0 kg/m2 were positively associated with in-hospital mortality in large artery stroke (2.42 (1.26−4.65)) (Table 2).
Hemorrhagic stroke
The clinical characteristics of patients with ICH or SAH according to the BMI categories are presented in Table S6–7, respectively. Among those with ICH, patients with underweight exhibited larger hematoma volumes, and lobar ICH and less often in deep ICH than in patients with obesity. Figure S4 shows the proportions of individuals with mRS scores among those with ICH and SAH. An inverse linear trend was observed between unfavorable outcomes and the BMI categories in those with ICH (p for trend <0.001) and SAH (p for trend = 0.017). In the multivariable analysis, compared to patients with normal weight, underweight patients with ICH were associated with unfavorable outcomes (1.41 (1.01−1.99)) and a shift toward unfavorable outcomes (1.31 (1.08−1.61)); this effect was not seen in those with SAH. The inverse association between being underweight and favorable outcomes was observed in patients with ICH (0.70 (0.49−0.99)) (Table S8). In the adjusted restricted cubic spline analysis, lower BMI levels (<23) were associated with a higher risk of unfavorable outcomes in patients with ICH (Figure 2(c)).
The analysis using traditional BMI categories is shown in the Supplemental result and Table S9–10.
Discussion
In this registry-based study of acute stroke, being underweight was consistently associated with higher risks of unfavorable functional outcomes after IS and ICH, but not after SAH. One of the novel findings was the association between BMI and post-stroke disability after specific IS subtypes and ICH. In patients with overall IS or small-vessel occlusion, being overweight was associated with lower frequency of unfavorable outcomes compared with the outcomes in those with normal weight. Patients with large artery stroke exhibited a U-shaped association between BMI categories and the likelihood of in-hospital mortality; patients with a BMI ⩾30.0 kg/m2 was another high-risk factor for mortality. The subgroup analyses demonstrated that lower frequency of unfavorable outcomes was observed in those with obesity; this association was significant in older patients (age ⩾80 years) with IS than in younger ones. In addition, the effect of being underweight on unfavorable outcomes was apparent in patients with higher NIHSS (⩾10).
The observed associations between underweight and unfavorable outcomes and mortality after IS were consistent with the results of a meta-analysis based on data from 14 studies that supported the existence of the “lean paradox.” 6 Although the cause of the lean paradox remains unclear, it may be driven by systemic catabolic activities, such as lipolysis and proteolysis, which are exacerbated by the hyperglycemia and weight loss that commonly occur after acute stroke.15,16 Poor nutritional status and weight loss are related to an increased stress response, a higher frequency of infections, and impaired stroke recovery. 15 Overweight patients may be less impacted by these changes, as they have greater metabolic reserves that can reduce the risk of cachexia and increase the likelihood of surviving post-stroke complications. Furthermore, patients with higher BMI tended to be younger in age, with more frequent metabolic risk profiles (hypertension, diabetes, dyslipidemia), consistent with the findings of previous studies.17–20 In contrast, underweight patients were a decade older, with a greater prevalence of previous stroke and cancer compared with those with obesity. The impact of BMI on post-stroke recovery may result from pre-existing systemic insufficiencies or characteristics. Although we performed risk adjustment for multiple confounding factors, other potential factors (e.g. frailty, sarcopenia, or socioeconomic status) that be unaccounted for may explain this paradox. A possibility could be related to the fact that the patients with underweight had the highest initial NIHSS score among the BMI categories in this study, potentially limiting the opportunity for favorable functional outcomes. However, the severity of neurological deficits as measured by NIHSS scores across BMI categories has not been consistently reported across previous studies.17,18,20
The protective effect of obesity on post-IS disability and mortality, the so-called “obesity paradox” that was demonstrated in a systematic review, 21 was not observed for IS subtypes in this study; in fact, obesity was positively associated with mortality in those with large artery stroke. This discrepancy may have been a result of differences in the follow-up period between studies; 21 the obesity paradox was only evident after a sufficient amount of time had elapsed after stroke onset. 22 Conversely, in terms of cardiac outcomes, the paradox was more evident during the short-term follow-up period and emerged as higher long-term mortality among obese patients. 23
In this study, patients with large artery stroke exhibited a U-shaped association between BMI and unfavorable outcomes. Although such an association has already been reported in patients with coronary artery disease,23,24 there is still a paucity of data supporting such a relationship in patients with stroke. Furthermore, a differential effect of obesity on outcomes depended on its severity, with the apparently protective effects limited to those who were overweight or had mild obesity and were absent in those with severe obesity.18,25 A recent study involving 1033 patients with acute IS from the Field Administration of Stroke Therapy–Magnesium (FAST-MAG) trial showed that the OR of unfavorable functional outcomes (mRS 2–6) was highest in the extreme BMI groups (BMI < 18.5 or > 35.0 kg/m2) and lowest in those with a BMI of approximately 25 kg/m2 in the unadjusted analysis; however, the relationship was no longer present after adjustment for certain covariates, including the NIHSS score. 17
In patients with ICH, being underweight was associated with unfavorable functional outcomes, but not in-hospital mortality. In contrast, most previous studies focused on and were in support of an inverse relationship between BMI and mortality after ICH.26,27 The likelihood of an unfavorable outcome among patients with SAH did not vary across BMI categories, which is in agreement with previous studies.6,28 A relationship between functional outcomes and BMI in the unadjusted analyses was diminished after accounting for the initial stroke severity, which indicated that the strong prognostic determinant factors included the initial stroke severity in those with acute SAH. In contrast, higher BMI values were shown to be associated with poor outcomes in patients with SAH who underwent surgical intervention. 29 The disparate results between studies are likely due to differences in patient characteristics and sample sizes, making it difficult to draw definitive conclusions.
A strength of this study was the substantially large sample size, which enabled analyses based on individual IS subtypes with sufficient statistical power. This study also had inherent limitations. First, body weight and height were both assessed in only 32% of patients in the data from the JSDB, partly due to the difficulty in ensuring that accurate measurements are conducted in an acute setting, especially for those with severe stroke. Height and body weight were measured in different clinics using different scales. Not all equipment be calibrated in the same way nor correctly. Thus, selection bias could be considered due to the lack collection of consecutive BMI data, which limits the strength of association and generalizability. Second, BMI was based on a single assessment conducted at baseline; therefore, the potential influence of BMI changes over time on prognosis could not be assessed. Third, although BMI is widely used for assessment in epidemiological and clinical settings, BMI is not an accurate estimate of body composition and reflects of both adipose and muscle mass. Fourth, outcome measures were assessed at the time of discharge. Fifth, data on lifestyle-related variables, such as physical activity, and the incidence of certain complications (e.g. aspiration pneumonia, urinary tract infection, and sepsis) during hospitalization were not available for analysis in this study. Sixth, all data were collected solely from Japanese patients, which could limit the generalizability of the findings to other ethnicities.
This study demonstrates the clinical impact of BMI on post-stroke recovery, and the identification of patients with different stroke prognoses will improve outcomes in clinical practice. Ultimately, maintaining an optimal body weight is required to ensure favorable post-stroke outcomes. Furthermore, given the dramatic increase in the proportion of older individuals in the general population, strategic attempts should be made to prevent muscle mass and nutritional deficiencies related to being underweight, which could, in turn, mitigate the risk of poorer outcomes in later life.
Supplemental Material
Supplemental material, sj-docx-1-wso-10.1177_17474930241249370 for Clinical impact of body mass index on outcomes of ischemic and hemorrhagic strokes by Kaori Miwa, Michikazu Nakai, Sohei Yoshimura, Yusuke Sasahara, Shinichi Wada, Junpei Koge, Akiko Ishigami, Yoshiki Yagita, Kenji Kamiyama, Yoshihiro Miyamoto, Shotai Kobayashi, Kazuo Minematsu, Kazunori Toyoda and Masatoshi Koga in International Journal of Stroke
Acknowledgments
The authors thank Ms. Ai Ito for secretary assistant. A list of Japan Stroke Data Bank (JSDB) co-investigators is given in the Supplemental Material (Appendix 1).
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Grants-in-Aid for Scientific Research (21K07472) and (19K19373).
ORCID iDs: Kaori Miwa  https://orcid.org/0000-0002-7948-3636
https://orcid.org/0000-0002-7948-3636
Junpei Koge  https://orcid.org/0000-0002-9632-2164
https://orcid.org/0000-0002-9632-2164
Kazuo Minematsu  https://orcid.org/0000-0003-3307-2708
https://orcid.org/0000-0003-3307-2708
Kazunori Toyoda  https://orcid.org/0000-0002-8346-9845
https://orcid.org/0000-0002-8346-9845
Masatoshi Koga  https://orcid.org/0000-0002-6758-4026
https://orcid.org/0000-0002-6758-4026
Data availability: The data are available from the corresponding author upon reasonable request.
Supplemental material: Supplemental material for this article is available online.
References
- 1. Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation 2022; 145: e153–e639. [DOI] [PubMed] [Google Scholar]
- 2. GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 2021; 20: 795–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 2013; 309: 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Antonopoulos AS, Tousoulis D. The molecular mechanisms of obesity paradox. Cardiovasc Res 2017; 113: 1074–1086. [DOI] [PubMed] [Google Scholar]
- 5. Andersen KK, Olsen TS. The obesity paradox in stroke: lower mortality and lower risk of readmission for recurrent stroke in obese stroke patients. Int J Stroke 2015; 10: 99–104. [DOI] [PubMed] [Google Scholar]
- 6. Zhang P, Yan XL, Qu Y, Guo ZN, Yang Y. Association between abnormal body weight and stroke outcome: a meta-analysis and systematic review. Eur J Neurol 2021; 28: 2552–2564. [DOI] [PubMed] [Google Scholar]
- 7. Kobayashi S, Fukuma S, Ikenoue T, Fukuhara S, Kobayashi S. Effect of edaravone on neurological symptoms in real-world patients with acute ischemic stroke. Stroke 2019; 50: 1805–1811. [DOI] [PubMed] [Google Scholar]
- 8. Toyoda K, Yoshimura S, Nakai M, et al. Twenty-year change in severity and outcome of ischemic and hemorrhagic strokes. JAMA Neurol 2022; 79: 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miwa K, Koga M, Nakai M, et al. Etiology and outcome of ischemic stroke in patients with renal impairment including chronic kidney disease: Japan Stroke Data Bank. Neurology 2022; 98: e1738–e1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24: 35–41 [DOI] [PubMed] [Google Scholar]
- 11. Lloyd-Jones DM, Allen NB, Anderson CAM, et al. Life’s essential 8: updating and enhancing the American Heart Association’s construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation 2022; 146: e18–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363: 157–163. [DOI] [PubMed] [Google Scholar]
- 13. Schlegel D, Kolb SJ, Luciano JM, et al. Utility of the NIH Stroke Scale as a predictor of hospital disposition. Stroke 2003; 34: 134–137. [DOI] [PubMed] [Google Scholar]
- 14. Rundek T, Mast H, Hartmann A, et al. Predictors of resource use after acute hospitalization: the Northern Manhattan Stroke Study. Neurology 2000; 55: 1180–1187. [DOI] [PubMed] [Google Scholar]
- 15. Dávalos A, Ricart W, Gonzalez-Huix F, et al. Effect of malnutrition after acute stroke on clinical outcome. Stroke 1996; 27: 1028–1032. [DOI] [PubMed] [Google Scholar]
- 16. Jönsson AC, Lindgren I, Norrving B, Lindgren A. Loss after stroke: a population-based study from the Lund Stroke Register. Stroke 2008; 39: 918–923. [DOI] [PubMed] [Google Scholar]
- 17. Liu Z, Sanossian N, Starkman S, et al. Adiposity and outcome after ischemic stroke: obesity paradox for mortality and obesity parabola for favorable functional outcomes. Stroke 2021; 52: 144–151. [DOI] [PubMed] [Google Scholar]
- 18. Zhao L, Du W, Zhao X, et al. Favorable functional recovery in overweight ischemic stroke survivors: findings from the China National Stroke Registry. J Stroke Cerebrovasc Dis 2014; 23: e201–e206. [DOI] [PubMed] [Google Scholar]
- 19. Doehner W, Schenkel J, Anker SD, Springer J, Audebert HJ. Overweight and obesity are associated with improved survival, functional outcome, and stroke recurrence after acute stroke or transient ischaemic attack: observations from the TEMPiS trial. Eur Heart J 2013; 34: 268–277. [DOI] [PubMed] [Google Scholar]
- 20. Vemmos K, Ntaios G, Spengos K, et al. Association between obesity and mortality after acute first-ever stroke: the obesity-stroke paradox. Stroke 2011; 42: 30–36. [DOI] [PubMed] [Google Scholar]
- 21. Oesch L, Tatlisumak T, Arnold M, Sarikaya H. Obesity paradox in stroke—myth or reality? A systematic review. PLoS ONE 2017; 12: e0171334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim BJ, Lee SH, Jung KH, et al. Dynamics of obesity paradox after stroke, related to time from onset, age, and causes of death. Neurology 2012; 79: 856–863. [DOI] [PubMed] [Google Scholar]
- 23. Wang ZJ, Zhou YJ, Galper BZ, Gao F, Yeh RW, Mauri L. Association of body mass index with mortality and cardiovascular events for patients with coronary artery disease: a systematic review and meta-analysis. Heart 2015; 101: 1631–1638. [DOI] [PubMed] [Google Scholar]
- 24. Ma WQ, Sun XJ, Wang Y, Han X-Q, Zhu Y, Liu N-F. Does body mass index truly affect mortality and cardiovascular outcomes in patients after coronary revascularization with percutaneous coronary intervention or coronary artery bypass graft? A systematic review and network meta-analysis. Obes Rev 2018; 19: 1236–1247. [DOI] [PubMed] [Google Scholar]
- 25. Ortega FB, Lavie CJ, Blair SN. Obesity and cardiovascular disease. Circ Res 2016; 118: 1752–1770. [DOI] [PubMed] [Google Scholar]
- 26. Cao Z, Liu X, Li Z, et al. Body mass index and clinical outcomes in patients with intracerebral haemorrhage: results from the China Stroke Center Alliance. Stroke Vasc Neurol 2021; 6: 424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Persaud SR, Lieber AC, Donath E, et al. Obesity paradox in intracerebral hemorrhage. Stroke 2019; 50: 999–1002. [DOI] [PubMed] [Google Scholar]
- 28. Hughes JD, Samarage M, Burrows AM, Lanzino G, Rabinstein AA. Body mass index and aneurysmal subarachnoid hemorrhage: decreasing mortality with increasing body mass index. World Neurosurg 2015; 84: 1598–1604. [DOI] [PubMed] [Google Scholar]
- 29. Rautalin I, Juvela S, Macdonald RL, Korja M. Body mass index and the risk of poor outcome in surgically treated patients with good-grade aneurysmal subarachnoid hemorrhage. Neurosurgery 2022; 90: 816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-wso-10.1177_17474930241249370 for Clinical impact of body mass index on outcomes of ischemic and hemorrhagic strokes by Kaori Miwa, Michikazu Nakai, Sohei Yoshimura, Yusuke Sasahara, Shinichi Wada, Junpei Koge, Akiko Ishigami, Yoshiki Yagita, Kenji Kamiyama, Yoshihiro Miyamoto, Shotai Kobayashi, Kazuo Minematsu, Kazunori Toyoda and Masatoshi Koga in International Journal of Stroke